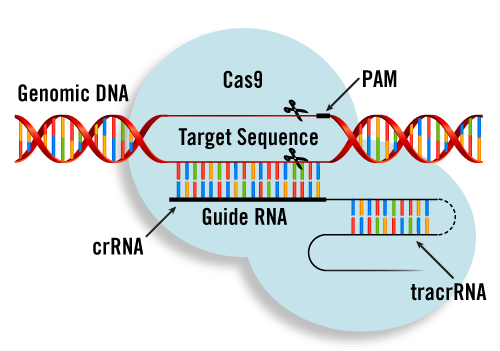
The PMWC 2022 Emerging Therapeutics Showcase will provide a 15-minute time slot for selected companies and researchers in the CRISPR, Cell and Gene Therapy fields. Major advancements in safer cell- and gene-level editing technologies are bringing us closer toward cures for life-threatening disorders, from cancer to HIV to Huntington’s disease. Cell therapy, in which cellular material such as T cells capable of fighting cancer cells, is injected into a patient, has been demonstrated safe and effective. The popular new CRISPR tool that has been used to edit the genetic code of nearly any organism will have an enormous impact on human health. More than a dozen clinical trials employing CRISPR on human cells are already underway.
- Emerging Therapeutics/Cell Therapy
- Emerging Therapeutics/CVD
- Emerging Therapeutics/Gene Therapy
- Emerging Therapeutics/Immunotherapy
- Emerging Therapeutics/Radiation Therapy
Confirmed Presenting Companies:
Session Chair Profile
Biography
Daniel S. Chen, MD, PhD, is a Director on the Society for ImmunoTherapy of Cancer (SITC) Board of Directors, the former Chief Medical Officer for IGM Biosciences, and former Vice President, Global Head of Cancer Immunotherapy Development at Genentech/Roche. He is a reviewer for Nature, Immunity and Clinical Cancer Research, serves on the Board of Directors for SITC, gave the keynote presentation at the AACR NCI EORTC Annual Meeting 2014 and presented at the US Congressional Briefing on Immuno Oncology in 2017. He has continued to publish with academic and industry collaborators in the field of cancer immunotherapy, including the often-referenced Chen and Mellman manuscripts, “Oncology meets Immunology: the Cancer-Immunity Cycle,” “Elements of cancer immunity and the cancer-immune set point” and the Hegde and Chen manuscript “Top 10 Challenges in Cancer Immunotherapy.”
Speaker Profile
Biography
Blake Wise is Chief Executive Officer at Novome Biotechnologies, a biotechnology company focused on developing engineered cellular therapies for the gut to treat chronic diseases. Prior to Novome, Mr. Wise served as Chief Executive Officer of Achaogen where he oversaw the development and FDA approval of ZEMDRI (plazomicin) for adults with complicated urinary tract infections. Prior to joining Achaogen, Mr. Wise spent 13 years at Genentech in multiple leadership positions of increasing responsibility, including Vice President in Genentech’s BioOncology business unit. Prior to joining Genentech, Mr. Wise worked in consumer marketing, e-commerce and online marketing in leadership positions at Gap, Inc. and Webvan. Mr. Wise received a Bachelor of Arts degree in Business Economics from University of California, Santa Barbara, and a Masters of Business Administration degree from University of California, Berkeley, Haas School of Business.
Emerging Therapeutics Showcase:
Novome Biotechnologies
Novome Biotechnologies is a clinical stage biotechnology company developing engineered cellular therapies for the gut to treat chronic diseases.
Engineering Living Medicines for Chronic Diseases
In this talk, we discuss Novome’s novel foundational technology for controllably colonizing the gut with therapeutically engineered cells, our broad pipeline, and clinical results from a Phase 1 healthy volunteer study of our lead program, NOV-001.
Speaker Profile
Biography
Dr. Behl specializes in lung cancer, melanoma and head and neck cancers. Dr. Behl leads clinical research and has coauthored peer-reviewed articles and published in high-impact journals such as the Journal of Clinical Oncology (JCO) and Chest.
Emerging Therapeutics Showcase:
Sutter Health
Not Just Any EGFR Mutation: The Importance of Testing for Exon 20
Patients with metastatic NSCLC with EGFR exon 20 insertion mutations have poorer outcomes than those patients who have more common EGFR mutations. Because >100 different EGFR exon 20 insertion mutations have been identified, comprehensive biomarker testing by NGS is recommended
Speaker Profile
Biography
Edison Hudson is a recognized pioneer in robotics and intelligent process automation. At Panacea BioMatx Inc, he has led the design of disruptive robotic platforms for personalized medicine and clinical nutrition driven by digital health data. He has been a key innovator in several technology companies, including iRobot, Adept Technology, Unimation, Redzone Robotics, and Meta Machines, and spent over a decade working in Silicon Valley automating semiconductor and electronics manufacturing processes. Founder of 4 companies, he holds over 25 patents in automation, machine intelligence, and automated pharmacy. A Morehead Scholar at the University of North Carolina, he studied artificial intelligence at Oxford University UK, and received an MBA from Duke University.
Wellness & Aging Showcase:
OneFul Health Inc
OneFul seeks to improve the quality of life for millions of patients with affordable personalized medicine that are easier to take. Science-based formulations, made with robotic accuracy, deliver multiple drugs combined for One Person, as prescribed by One Doctor from our accredited OneFul Pharmacies.
Personalized Cardiovascular Therapies - Genomics Meets Robotics
Fixed combination cardiovascular poly-pills have been shown to greatly improve adherence in multiple clinical studies. OneFul shows how pharmacogenomic-based formulation driving highly accurate robotics can make personalized CVD poly-therapies that are more efficacious with fewer adverse effects, further enhancing poly pharmacy adherence.
Speaker Profile
Biography
Marek has obtained his PhD at the Cold Spring Harbor Laboratory working in prof. Gregory J. Hannon lab. He was working on custom genomics approaches in the small RNA biology research. He subsequently followed with a postdoc at the University of California, Berkeley with prof. Nick Ingolia studying the regulation of translation using bioinformatics and deep learning tools.
Emerging Therapeutics Showcase:
Ardigen
Ardigen is harnessing advanced AI methods for novel precision medicine. The company’s in-house datasets, together with advanced AI platforms, empower the development of effective therapies.
AI-aided Rational Design of Cancer Cell Therapies
Predicting the interaction of a TCR to target and off-target antigens can support the development of adoptive cell therapies. We will discuss how AI-assisted methods can help by incorporating sequence-level information and general patterns of interactions at the peptide, HLA, and TCR level.
Speaker Profile
Biography
Executive level leadership with extensive experience in drug development, clinical science, translational medicine and overseeing development strategies for early to late stage assets. Initiation and execution on Academic Alliances and Partnerships. Known for versatility and unique ability to extend skills across diverse functional roles, including strategic planning, product development, brand strategies, ability to develop trusted relationships with both internal and external partners, exceptional medical and scientific knowledge with a broad understanding of disease entities and corresponding molecular and business implications.
Emerging Therapeutics Showcase:
3TBiosciences
T cell receptor-based therapeutics company against novel targets for cancer and other immune-related diseases. The platform combines high diversity libraries and active machine learning to discover novel tumor-specific targets and assess the specificity and cross-reactivity of a potential TCR therapeutic.
Identification of Novel pHLA Targets for Solid Tumor
Speaker Profile
Biography
Brad Niles received his Ph.D. from UC Davis, with a Designated Emphasis in Biotechnology and advanced training in Business Development. He has 15 years of experience in cancer research, and has published more than a dozen scientific papers. In addition, he has managed product development projects for two biotech startups, and is an inventor on numerous RNA-therapeutic patent applications. At ARIZ he has previously served as COO and VP of Research and Development for over 4 years.
Emerging Therapeutics Showcase:
ARIZ Precision Medicine
ARIZ Precision Medicine is focused on the epigenetic changes that occur in the cancerization of a cell to develop targeted therapeutics. With an innovative approach that utilizes the differences in cancer cells, we can specifically kill cancer cells while sparing healthy cells.
PRDM2: A Druggable Target in Lung Cancer
ARIZ is developing a first in class siRNA therapy for lung cancer, targeting a driver gene that is misregulated in 75% of lung cancers with an siRNA packaged in a drug delivery system that achieves significantly tumor growth inhibition.
Speaker Profile
Biography
Scott recently joined CDD to help refine and grow its new BioHarmony data services business. Scott has over 30 years of experience in the pharmaceutical research industry. His career has focused on the development and commercialization of informatics tools and services that improve the drug discovery & development process. Scott has served in multiple senior leadership roles including VP, GM at Tripos, Inc. a Computer Aided Molecular Design company, Co-Founder & CEO of ChemNavigator.com an online chemistry services company which was acquired by Sigma-Aldrich Inc., and Commercial Director of Elsevier Life Sciences Professional Services.
AI and Data Sciences Showcase:
Collaborative Drug Discovery
CDD advances science through better data management. It’s CDD Vault® product, is a hosted informatics solution that allows researchers to organize data and experiments and securely collaborate in real time. CDD also offers BioHarmony™ Drug Data Store and Annotator an ML assisted bioassay annotation platform.
ML Bioassay Annotation and FAIR Drug Data
FAIR Drug data and new ML technology for the automated curation and annotation of bioassay protocols.
Speaker Profile
Biography
Hubert Chen is SVP of Clinical Development at Krystal Biotech, where he leads a growing portfolio of clinical-stage programs utilizing Krystal’s HSV-1–based platform to enable the development of redosable gene therapies for rare, life-threatening conditions, including dystrophic epidermolysis bullosa and cystic fibrosis. Hubert is a physician-scientist with over a decade of industry experience spanning all phases of drug development from first-in-human to launch. Previously, Hubert was Principal Medical Director at Genentech where he led the FDA approval for omalizumab in chronic spontaneous urticaria and was responsible for the advancement of multiple early-stage programs in the respiratory/immunology pipeline. Hubert began his career as an assistant professor in the pulmonary division at UCSF and has published over 40 peer-reviewed articles in the field of respiratory medicine. He is board-certified in pulmonary and critical care medicine. He received his MD from Stanford, his MPH from Harvard, and completed his fellowship at UCSF.
Emerging Therapeutics Showcase:
Krystal Biotech
Krystal Biotech, Inc. (NASDAQ: KRYS) is a pivotal-stage gene therapy company leveraging its proprietary, redosable gene therapy platform and in-house manufacturing capabilities to develop life-changing medicines for patients with serious diseases, including rare diseases in skin, lung, and other areas.
Redosable Gene Therapies Utilizing a HSV-1-based Platform
Krystal Biotech has created a proprietary HSV-1–based platform, enabling development of redosable gene therapies.