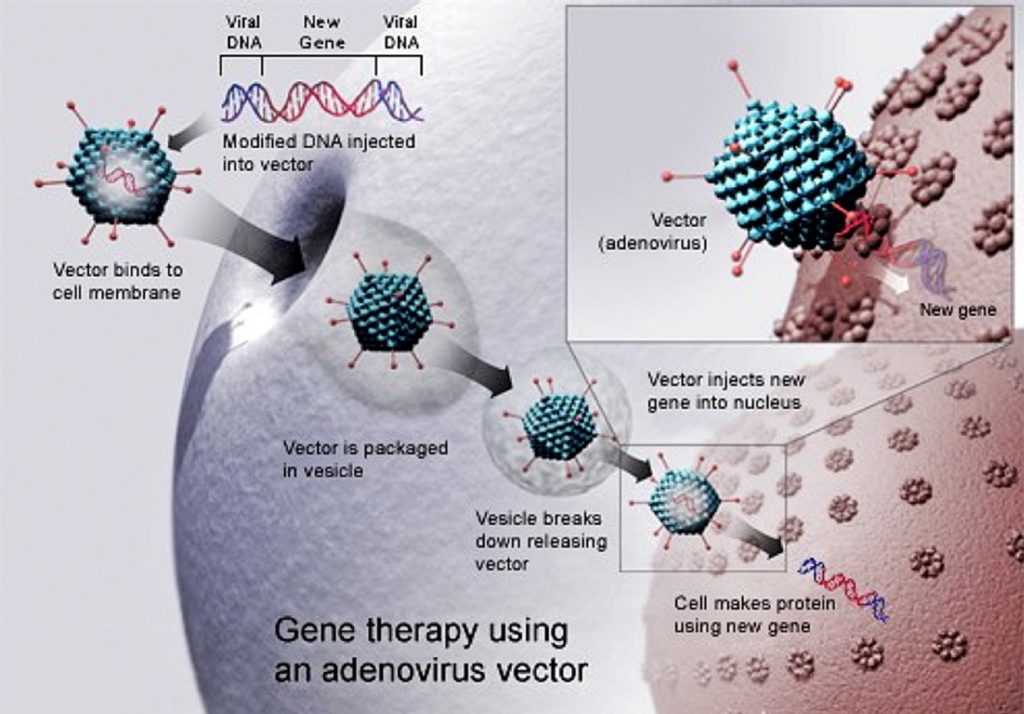
Track Chair: Sharon Benzeno, Adaptive Biotechnologies
- PMWC 2023 Luminary Award Ceremony
Honoree: Bruce Levine, University of Pennsylvania
- Advance and Optimize Cell Therapies in Oncology (PANEL)
Chair: Sharon Benzeno, Adaptive Biotechnologies
- Ira Mellman, Genenetch
- Mark Selby, Walking Fish Therapeutics
- Sneha Ramakrishna, Stanford
- Loïc VINCENT, Affini-T Therapeutics
- Nick Haining, Arsenal
- Warner Biddle, Kite - Pioneering Cell Therapies in the Treatment of Autoimmune Disorders (PANEL)
Chair: Carl June, UPenn
- Lenny Dragone, Sonoma
- James Chung, Kyverna
- David Chang, Cabaletta
- Cristina Musselli, Abata Therapeutics - Innovations in Cell Therapy Manufacturing (PANEL)
Chair: Bruce Levine, University of Pennsylvania
- Chris Holt, BMS
- Heidi Hagen, Sonoma Bio
- Rahul Singhvi, Resilience
- Snehal Patel, Sana Biotechnology - Patentability of Immunotherapies - Latest Developments
- Janet Xiao, Morrison & Foerster LLP
- Investing in Gene-Modified Cell Therapies
Chair: Karen Tkach Tuzman, BioCentury Inc.
- Robert Lin, UPMC
- Yvonne Yamanaka, venBio
- Abraham Bassan, Samsara Capital
- Brian Daniels, 5am Ventures
- Leveraging Data Sets to Inform Future Drug Discovery and Development (PANEL)
Chair: Jason Coloma, Maze Therapeutics
- Peter DiLaura, Sonoma Bio
- Nick Haining, Arsenal
- Kwame Okrah, Parker Institute
- Dan Kirouac, Notch Therapeutics
Session Chair Profile
Biography
Dr. Jason Coloma is the CEO of Maze Therapeutics. Prior to his appointment to CEO, he was a venture partner at Third Rock Ventures, where he was instrumental in the founding and launch of the company. Previously, he held a number of roles at Roche, including vice president & global therapeutic area head of oncology and cancer immunotherapy partnering. Before joining Roche, Jason was a consultant in the life sciences practice at L.E.K. Consulting. He also worked in finance at Amgen and in research at the University of California, San Francisco and Cytokinetics.
Jason holds a Ph.D. and M.P.H. at the University of California, Berkeley, an MBA from the Tuck School of Business at Dartmouth and a B.S. in biology from the University of San Francisco.
Session Chair Profile
Biography
As Head of Discovery and Preclinical Research, Karen guides BioCentury’s coverage of emerging biology and technologies. She has written extensively about immunology, synthetic biology and data science, covering innovative breakthroughs in industry and academia, and dissecting trends in company formation and investments around new science.
Karen joined BioCentury in 2015 after a postdoctoral research fellowship in Stanford University’s Department of Chemical and Systems Biology. She holds a Ph.D. in Immunology from Weill Cornell Medicine and a B.A. in Molecular and Cell Biology from the University of California Berkeley.
Speaker Profile
Biography
Ira Mellman came to Genentech in the Spring of 2007 as Vice President of Research Oncology, after more than 20 years as a faculty member at the Yale University School of Medicine, where he was chair of his department (Cell Biology), a member of the Ludwig Institute for Cancer Research, scientific director of the Yale Cancer Center, and Sterling Professor of Cell Biology and Immunobiology. Dr. Mellman has a BA from Oberlin College & Conservatory and a PhD in Genetics from Yale. He was a Postdoctoral Fellow at the Rockefeller University with Ralph Steinman, who received the Nobel Prize for the discovery of dendritic cells. His laboratory is known not only for advances in fundamental cell biology particularly in the area of membrane traffic (including the discovery of “endosomes”) but also for applying these insights to understanding the cellular basis of the immune response, especially dendritic cell function. He was also the founder of CGI Pharma, which was recently purchased by Gilead. Ira ran all of oncology research at Genentech until the end of 2013 when he decided to concentrate his efforts on the rapidly developing area of cancer immunotherapy and became Vice President of Cancer Immunology. Ira is a member of the National Academy of Sciences, American Academy of Arts & Sciences, the European Molecular Biology Organization, and the former Editor in Chief of the Journal of Cell Biology. He has also served on the editorial boards of Cell, the Journal of Experimental Medicine, EMBO Journal, among others. He also serves on the boards of the Society for the Immunotherapy of Cancer, the American Society for Cell Biology, and the Cancer Research Institute. He remains a frustrated composer and songwriter, and has recorded two CDs in the little-known genre of “bio-rock”.
Speaker Profile
Biography
Gautam is a leader in translational computational biology and a seasoned professional with an impactful 20-year track record in biotech, academia and tech industry. Most recently, Gautam built and headed an inter-disciplinary R&D/Consulting practice of five Ph.D. computational biologists to implement pipelines for analysis of multi-omics datasets including single-cell RNA-seq, CyTOF, proteomics and metabolomics from cross-sectional/longitudinal observation/intervention studies. Gautam advised biotech and pharmaceutical companies on challenges in early-stage target discovery, biomarker analysis and patient stratification for clinical development, and identification of mechanisms of action and resistance to treatments. Prior to that, he spearheaded biomarker research and platform development as Director of Precision Medicine at a biotech to accelerate clinical development of Antigen-specific Immunotherapies. He led the biomarker discovery program in Celiac Disease which resulted in the 1st blood-based diagnostic and an immune response monitoring toolkit to support Phase 2 clinical trials.
Speaker Profile
Biography
Janet focuses on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies. Her clients range from large multinational biopharmaceutical companies, such as Celgene and Genentech, to emerging startup companies around the world. She advises her clients on patent matters relating to various technologies, including antibody therapeutics, cell therapeutics, nanomedicine, gene therapy, drug delivery systems, diagnostics, and nutraceuticals. Janet works extensively in performing IP due diligence reviews in the contexts of VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. She is noted for her in-depth knowledge of patent landscapes in the fields of cancer immunotherapy, gene editing, next-generation sequencing, liquid biopsy, antibody manufacturing, and medical devices.
Talk
This discussion will focus on the latest patent law developments in the field of immunotherapy including patentability issues, recent case law, and patent strategy considerations. Participants will gain insight on how to navigate the evolving case law around immunotherapies and build valuable patent portfolios in light of these new developments.
Session Chair Profile
Biography
Dr. Bruce Levine, Barbara and Edward Netter Professor in Cancer Gene Therapy, is the Founding Director of the Clinical Cell and Vaccine Production Facility (CVPF) in the Department of Pathology and Laboratory Medicine and the Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania. He received a B.A. (Biology) from Penn and a Ph.D. in Immunology and Infectious Diseases from Johns Hopkins. First-in-human adoptive immunotherapy trials include the first use of a lentiviral vector, the first infusions of gene edited cells, and the first use of lentivirally-modified cells to treat cancer. Dr. Levine is co-inventor of the first FDA approved gene therapy (Kymriah), chimeric antigen receptor T cells for leukemia and lymphoma, licensed to Novartis. Dr. Levine is co-inventor on 30 issued US patents and co-author of >200 manuscripts and book chapters with a Google Scholar citation h-index of 100. He is a Co-Founder of Tmunity Therapeutics, and of Capstan Therapeutics both spinouts of the University of Pennsylvania. Dr. Levine is a recipient of the William Osler Patient Oriented Research Award, the Wallace H. Coulter Award for Healthcare Innovation, the National Marrow Donor Program/Be The Match ONE Forum 2020 Dennis Confer Innovate Award, serves as Immediate Past-President of the International Society for Cell and Gene Therapy, and serves on the Board of Directors of the Alliance for Regenerative Medicine. He has written for Scientific American and Wired and has been interviewed by the NY Times, Wall Street Journal, Washington Post, NPR, Time Magazine, National Geographic, Bloomberg, Forbes, BBC, and other international media outlets.
Session Chair Profile
Biography
Dr. Sharon Benzeno is the Chief Commercial Officer, Immune Medicine and leads the Drug Discovery group of Adaptive Biotechnologies, a commercial-stage biotech company that aims to translate the genetics of the adaptive immune system into clinical products to diagnose and treat disease. Before joining Adaptive in 2014, Sharon was Senior Director at Elsevier Inc., a healthcare informatics company. Prior to Elsevier, Sharon co-led the oncology business unit at Capgemini SE, a consulting and technology services company. Sharon came to Elsevier from Capgemini, where she served in a variety of management roles at AstraZeneca plc. Sharon holds a PhD in Biomedical Sciences from New York University School of Medicine, an MBA in Finance and Leadership from New York University Stern School of Business and a BA in Biochemistry from New York University. Sharon completed a postdoctoral fellowship in cancer biology at the University of Pennsylvania Abramson Cancer Center.
Speaker Profile
Biography
Lenny joined Sonoma Biotherapeutics from Janssen Biopharma where he served as the Vice President for Early Clinical Development as well as the interim Head of Data Sciences for Infectious Diseases. Lenny previously served as Senior Director of Experimental Medicine and Translational Pharmacology at Merck Research Laboratories in SSF. As clinical site lead and therapeutic area lead for Autoimmunity, Inflammation and Ophthalmology (AIO). Prior to this, Lenny started his career in industry at Genentech. Lenny received his degrees from the University of Rochester, before completing his Pediatric residency and Pediatric Rheumatology Fellowship training at UCSF. He then ran his own NIH-funded laboratory and advanced up the academic ranks to Associate Professor of Pediatrics and Immunology at the University of Colorado and National Jewish Health. Lenny continues to stay clinically active as a Volunteer Associate Professor of Pediatric Rheumatology at UCSF seeing patients in the pediatric rheumatology fellow’s clinic.
Speaker Profile
Biography
Dr. Bruce Levine, Barbara and Edward Netter Professor in Cancer Gene Therapy, is the Founding Director of the Clinical Cell and Vaccine Production Facility (CVPF) in the Department of Pathology and Laboratory Medicine and the Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania. He received a B.A. (Biology) from Penn and a Ph.D. in Immunology and Infectious Diseases from Johns Hopkins. First-in-human adoptive immunotherapy trials include the first use of a lentiviral vector, the first infusions of gene edited cells, and the first use of lentivirally-modified cells to treat cancer. Dr. Levine is co-inventor of the first FDA approved gene therapy (Kymriah), chimeric antigen receptor T cells for leukemia and lymphoma, licensed to Novartis. Dr. Levine is co-inventor on 30 issued US patents and co-author of >200 manuscripts and book chapters with a Google Scholar citation h-index of 100. He is a Co-Founder of Tmunity Therapeutics, and of Capstan Therapeutics both spinouts of the University of Pennsylvania. Dr. Levine is a recipient of the William Osler Patient Oriented Research Award, the Wallace H. Coulter Award for Healthcare Innovation, the National Marrow Donor Program/Be The Match ONE Forum 2020 Dennis Confer Innovate Award, serves as Immediate Past-President of the International Society for Cell and Gene Therapy, and serves on the Board of Directors of the Alliance for Regenerative Medicine. He has written for Scientific American and Wired and has been interviewed by the NY Times, Wall Street Journal, Washington Post, NPR, Time Magazine, National Geographic, Bloomberg, Forbes, BBC, and other international media outlets.
Speaker Profile
Biography
Chris leads day to day commercial CAR-T manufacturing operations at the BMS site in Bothell, delivering autologous therapies to patients globally.
He also works with the company’s larger cell therapy network to set the strategic direction for the site, to maximize the reach of commercially available cell therapies for patients and support the growth of its cell therapy pipeline.
Chris is a chemist, turned engineer with 25+ years of experience working in the Pharma, Biologics & CGT sectors. After starting and growing his career in Europe, he has spent the last 10 years in the US in a variety of leadership roles in manufacturing operations.
Session Chair Profile
Biography
Dr. Carl June studies mechanisms of lymphocyte activation that relate to immune tolerance and adoptive immunotherapy for cancer and chronic infection. In 2011, his research team published findings detailing a new therapy in which patients with refractory and relapsed chronic lymphocytic leukemia were treated with genetically engineered versions of their own T cells, CAR-Ts. The treatment has also now been used with promising results to treat children with refractory acute lymphoblastic leukemia. His work led to the development and commercialization of tisagenlecleucel, the first FDA-approved gene therapy. In the 1980s, his lab discovering the CD28 molecule as the major control switch for T cells. He has published more than 350 manuscripts and is the recipient of numerous prizes and honors, including election to the Institute of Medicine in 2012 and the American Academy of Arts and Sciences in 2014, the William B Coley award, the Richard V Smalley Memorial Award from the Society for Immunotherapy of Cancer, the AACR-CRI Lloyd J. Old Award in Cancer Immunology, the Philadelphia Award, the Taubman Prize for Excellence in Translational Medical Science, the Paul Ehrlich and Ludwig Darmstaedter Prize, the Novartis Prize in Immunology, the Karl Landsteiner Memorial award, the Debrecen Award and a lifetime achievement award from the Leukemia and Lymphoma Society. June received his Bachelor of Science in Biology from the United States Naval Academy and his M.D. from Baylor College of Medicine.
Speaker Profile
Biography
David J. Chang leads all clinical programs at Cabaletta Bio, which seeks to develop and launch the first, potentially curative, targeted cellular therapies for patients with autoimmune diseases. He is directing development of Cabaletta’s pipeline, including CABA-201, a fully human, 4-1BB containing CD19-CAR T cell product candidate, which has potential application across a broad range of autoimmune diseases such as SLE, rheumatoid arthritis, myositis, and systemic sclerosis. He is also leading programs with the highly specific Chimeric AutoAntibody Receptor T (CAART) approach, a precision therapy to eliminate disease-causing B cells, which is currently being evaluated in multiple clinical trials. Dr. Chang is a rheumatologist who received his medical education and training at NYU and Cornell and has >25 years of experience in immune-inflammation clinical development including as SVP of Inflammation, Autoimmunity and Neuroscience at AstraZeneca and in various senior positions at GSK. He also holds an adjunct professorship at the University of Pennsylvania.
Speaker Profile
Biography
Dr. Chung is responsible for developing and executing on the clinical strategy for Kyverna. Prior to Kyverna he was at Amgen where he began in Early Development and served as the Inflammation Therapeutic Area Head. He also worked in late-stage development as the Global Development Leader for Enbrel and as the Inflammation head for the US and Global Medical Organizations. Prior to Amgen he worked in early development, Inflammation, at Pfizer. Dr. Chung obtained his MD and PhD in Immunology at the University of Pennsylvania where he also completed his residency in Internal Medicine and fellowship in Rheumatology.
Speaker Profile
Biography
Rahul is a global leader in the Life Sciences industry. Most recently, he was an Operating Partner at Flagship Pioneering, where he was responsible for founding and operating companies launched from Flagship’s innovation foundry, Flagship labs. Before joining Flagship, Rahul was the Chief Operating Officer of Takeda’s Vaccine Business Unit where he was responsible for worldwide vaccine CMC and manufacturing operations. Before Takeda, Rahul was President and CEO of Novavax, Inc. where he transformed the company from a specialty pharmaceutical business to a vaccine development company. Rahul’s career began at Merck & Co in 1994, where he held several positions in R&D and manufacturing.Rahul serves on the Executive Advisory Board of the Leonard Davis Institute of Health Economics at the University of Pennsylvania and on the Scientific Advisory Board of the anti-microbial resistance research group at the Singapore MIT Advance Research and Technology program.
Speaker Profile
Biography
Mark has been dedicated to advancing immune-modulatory therapeutic options for patients. Currently, he is developing in vivo B cell protein factories providing durable expression of proteins of therapeutic value, exploiting the ability of B cells to produce immunoglobulin proteins at high levels and to persist for decades.
Mark started his industry career at Chiron Vaccines, working with Michael Houghton on exploring the HCV polyprotein for vaccines and subsequently on in vivo DNA delivery platforms to induce cellular immune responses. Thereafter, Mark worked with Dr. Alan Korman at Medarex, advancing fully human immune-modulatory antibodies derived from immunized transgenic mice. Mark is a co-inventor of the anti-PD-1 antibody Nivolumab (Opdivo), the first anti-PD-1 antibody to enter the clinic. Mark is co-inventor of the Nivolumab/Ipilumumab combination as well as the recently approved anti-LAG-3 antibody.
Speaker Profile
Biography
Loïc Vincent, PhD, is CSO at Affini-T Therapeutics. He is an oncology scientist with 20+ years’ international experience in academia / biotech / pharma industry, dedicated to advancing transformative therapies to patients suffering from cancer. Previously, Loïc was the Head of Takeda Oncology Drug Discovery Unit and Immunology Unit, and was responsible for leading Takeda Cell Therapy Discovery. Prior to joining Takeda in 2016, Loïc spent nine years at Sanofi, where he led Oncology Drug Discovery Pharmacology, Immunotherapy and Oncology External Innovation teams. Loïc is a former board member of the French-American Biotechnology Springboard and former Board Director at Adaptate Biotherapeutics. During his career, Loïc’s teams advanced more than 20 oncology drugs in the clinic.Loïc obtained his PhD in pharmacology from the University of Rouen, France. He carried out his postdoctoral fellowship in oncology at the Weill Medical College of Cornell University, NY.
Speaker Profile
Biography
Snehal has a breadth of knowledge and experience in leading roles in Technical Operations including Manufacturing, Technology, and Quality Assurance in Biologics, Small Molecules, and most recently in Cell Therapy. He is routinely sought out for his technical operational expertise, as well as his ability to collaborate and influence across all levels of the organization. In previous roles, Snehal has led a growing global manufacturing network to produce Clinical and Commercial Cell Therapy Products, including two cell therapies recently commercially launched in 2021. Prior to this role he was a Site Head for Cell Therapy Manufacturing. Earlier in his career, he held a variety of different roles with increasing responsibility, including Head of Global External Drug Product Manufacturing, Head of Drug Product Operations, and Head of Quality Operations.
Speaker Profile
Biography
Cristina has more than 15 years of biotech experience, beginning with Antigenics (now Agenus), a cancer immunotherapy company, where she worked many years at the bench to then move into clinical. She then joined Biogen where for the past 8 years, she’s been in the clinical development teamworking on a BDCA-2 antibody treatment for cutaneous lupus erythematosus whose results were recently published in the New England Journal of Medicine. She is currently the Head of Clinical Development, Senior Medical Director at Abata where she is supporting the development of autologous engineered Tregs for the therapy of MS.
Speaker Profile
Biography
Corporate development and strategy-focused senior executive, with leadership roles in range of discovery stage and clinical stage companies, and board member and advisor to multiple early-stage therapeutics companies. Prior to Sonoma Bio, Peter was an Entrepreneur-in-Residence at Third Rock Ventures, and has held leadership roles at multiple therapeutics and systems biology companies over his 25 year career in life sciences.
Speaker Profile
Biography
Nicholas Haining is a physician-scientist, immunologist and drug developer. He received his undergraduate and medical degree from Oxford University, UK. He completed his medical training in Pediatrics at Boston Childrens Hospital and in Pediatric Hematology/Oncology at Dana-Farber Cancer Institute.
As an Associate Professor of Pediatrics at Harvard Medical School and an Associate Member of the Broad Institute of MIT and Harvard, his lab defined some of the key transcriptional and epigenetic regulators of T cell exhaustion and used in vivo genetic screens to identify immune vulnerabilities of cancer cells in mouse models. His clinical expertise is in hematopoietic stem cell transplantation and he attended on the bone marrow transplant service at Boston Children’s Hospital for almost twenty years.
More recently, he served as Vice-President, Discovery Oncology and Immunology at Merck Research Laboratories, where he led a multi-site, multidisciplinary team developing innovative approaches to identify therapies for cancer and immune diseases.
Speaker Profile
Biography
Nicholas Haining is a physician-scientist, immunologist and drug developer. He received his undergraduate and medical degree from Oxford University, UK. He completed his medical training in Pediatrics at Boston Childrens Hospital and in Pediatric Hematology/Oncology at Dana-Farber Cancer Institute.
As an Associate Professor of Pediatrics at Harvard Medical School and an Associate Member of the Broad Institute of MIT and Harvard, his lab defined some of the key transcriptional and epigenetic regulators of T cell exhaustion and used in vivo genetic screens to identify immune vulnerabilities of cancer cells in mouse models. His clinical expertise is in hematopoietic stem cell transplantation and he attended on the bone marrow transplant service at Boston Children’s Hospital for almost twenty years.
More recently, he served as Vice-President, Discovery Oncology and Immunology at Merck Research Laboratories, where he led a multi-site, multidisciplinary team developing innovative approaches to identify therapies for cancer and immune diseases.
Speaker Profile
Biography
Dr. Kirouac leads the Systems Biology department at Notch Therapeutics, integrating bioinformatics, dynamical systems modelling and machine learning to design and deliver the next generation of T cell therapies. Prior to joining Notch he held scientific positions in large pharma, mid-size biotech and consulting, and has been developing mathematical models of biological systems for almost 20 years. Dr. Kirouac did post-doctoral training at MIT and Harvard Medical School, holds a PhD in Biomedical Engineering from the University of Toronto, and Bachelors in both Chemical Engineering and Genetics from the University of Western Ontario.
Talk
Speaker Profile
Biography
Kwame collaborates with scientists and clinicians across various organization to generate insights from clinical and biomarker data. His interests include the interplay between early stage drug development and bioinformatics, particularly how biomarker data can be used to tailor personalized cancer treatments for patients. Kwame previously worked as an early phase oncology biostatistician at Genentech, where he helped develop several early stage cancer immunotherapy molecules across various solid tumors. In addition to his clinical work, he collaborated with translational scientists to analyze high-throughput genomics biomarker data to inform the clinical pipeline and the design of new clinical trials. Kwame most recently formed his own consulting company, primarily focused on assisting small pharmaceutical companies that use T-cell engineering as a basis to fight cancer.
Speaker Profile
Biography
An accomplished operations executive with 30 years of experience in the biotechnology and medical device industries and an extensive track record in leading operations from early clinical trials through commercialization of innovative technologies ranging from recombinant protein/device combinations to the first active immune cell therapy, Provenge. She most recently served as interim Chief Executive Officer at Ziopharm Oncology (Alaunos), where she also sat on the company’s Board of Directors. Prior to Ziopharm, Hagen co-founded Vineti, a supply chain software company for cell and gene therapy, early on serving as the company’s Chief Strategy Officer. Before that, she served as interim Chief Commercial Officer at ZappRx, Global Chief Operating Officer at Sotio a.s., Senior Vice President Operations at Dendreon and Project Manager at Immunex. She has been responsible for operations including manufacturing, supply chain operations, and patient operations through clinical trials and commercialization. She sits on the boards of Obsidian Therapeutics and Vericel Corporation
Speaker Profile
Biography
Sneha Ramakrishna, M.D. obtained her B. A. from the University of Chicago and her M.D. from the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University. She completed her residency training in Pediatrics at the Children’s Hospital of Philadelphia and her fellowship in Pediatric Hematology/Oncology at the Johns Hopkins/National Cancer Institute combined program. Her research focuses on identifying mechanisms of relapse in patients following chimeric antigen receptor (CAR) T cell therapies and optimizing both CAR design and tumor sensitivity to improve long-term success of CAR T cell therapies. Clinically, Dr. Ramakrishna sees patients with pediatric solid tumors and treats children with CAR T cell therapies in the Cancer Cellular Therapies program.
Speaker Profile
Biography
Abe Bassan is a member of the investment team at Samsara BioCapital, a life science investment firm dedicated to building companies that advance innovative medicines. Prior to joining Samsara, he held operation roles at Revolution Medicines and Bluebird Bio, and before that was an Associate at Third Rock Ventures. Abe serves as a director on the boards of Graphite Bio, Link Cell Therapies, Septerna, Syncopation Life Sciences, and Vedere Bio II.
Speaker Profile
Biography
Yvonne Yamanaka is a Principal at venBio Partners with a background in biological engineering and life science company creation. Prior to joining venBio, Yvonne was a member of the venture creation team at Flagship Pioneering. She began her research career leading the development of a novel immunotherapy technology at EMD/Merck Serono. Yvonne received her Ph.D. in Biological Engineering from MIT and her B.S.E. in Biomedical Engineering from Duke University.
Speaker Profile
Biography
Rajul is a Managing Director at Vida Ventures and physician-scientist by background. Prior to coming to Vida, he was
most recently leading the Development organization at Kite Pharma, a Gilead company where he oversaw a team of
approximately 200 people developing engineered cell therapy products to treat a variety of blood cancers and solid
tumors. Rajul joined Kite in 2014 and was instrumental to the company’s growth and success, including the
advancement of Yescarta® and Tecartus® from pre-IND phase through global regulatory approvals.
Previously, Rajul was at Amgen where his last role was Global Development Lead. There he oversaw the development of
small molecules and biologics in the oncology and bone health therapeutic areas and was instrumental in advancing the
R&D pipeline including regulatory approvals of Xgeva and Prolia.
Rajul completed his BA in chemistry and biochemistry at Rice University, and MD and Internal Medicine internship and
residency at UT Southwestern Medical school, where he was a Howard Hughes Fellow. He completed his post-doctoral
training in biophysics at Rockefeller University, and fellowship training at MD Anderson Cancer Center, where he was
Chief Fellow.
Rajul serves on the Board of Directors of Capstan Therapeutics, InnoSkel, LocanaBio, Indupro, and Neogene
Therapeutics. Rajul was previously a volunteer Attending Physician treating underserved patients at UCLA Harbor
Medical Center and continues to lecture regularly at UCLA.
Speaker Profile
Biography
Brian Daniels joined 5AM Ventures as a Venture Partner in 2014 and transitioned to Partner in 2018. Dr. Daniels previously spent over two decades in clinical drug development, including leading the Development and Medical Affairs at Bristol-Myers Squibb for the last ten years. He directed the development of numerous innovative medicines that have contributed to the improvement for patients across a range of serious diseases. These include: ORENCIA and NULOJIX in immunology/transplant, REYATAZ, DAKLINZA and BARACLUDE in virology, ONGLYZA, FARXIGA and MYALEPT in metabolics, ELIQUIS in CV and YERVOY, OPDIVO, SPRYCEL and IXEMPRA in oncology. Dr. Daniels is currently a board director for Artiva Biotherapeutics and Inipharm and was previously on the board of Cabaletta Bio (NASDAQ: CABA). He is on the Scientific Advisory Boards for Soteria and Ideaya Biosciences (NASDAQ: IDYA). Dr. Daniels received B.S. and M.S. degrees from MIT and his M.D. from Washington University in St. Louis. He trained in internal medicine at New York Hospital and Rheumatology/Immunology at UCSF
Speaker Profile
Biography
Rob Lin, PhD, CFA, joined the UPMC Enterprises Translational Sciences team in 2017.
Dr. Lin is also CEO of Avista Therapeutics, a UPMC incubated company focused on ophthalmology gene therapy products. He was the Founding CEO of BlueSphere Bio and is currently on the board of directors of BlueSphere Bio, Code Biotherapeutics, Avista Therapeutics, and VegaVect, Inc.
Prior to working at UPMC, Dr. Lin led the finance and strategy team for the Global Health Program at the Bill & Melinda Gates Foundation. His previous experiences also include stints at Millennium Pharmaceuticals (now Takeda Oncology) and Dean and Company as a strategy consultant.
Dr. Lin earned a PhD in Genetics and an MMSc at Harvard University, as well as an MS in Chemical Engineering and a BS in Biological Sciences from Stanford University. He is a CFA Charterholder and previous Term Member of the Council on Foreign Relations (CFR).
Speaker Profile
Biography
Warner Biddle joined Kite in 2020.Before joining Kite, Warner served as Vice President for the BreastGynecologic and Skin Cancer Franchises at Genentech. There he successfully led the crossfunctional strategy and launches for several commercial and pipeline products while driving significant portfolio growth.Prior to his oncology roles, Warner served as Vice President, Sales and Marketing for Ophthalmology at Genentech and also held various global leadership roles in Europe and Canada across multiple therapeutic disease areas at Novartis and GlaxoSmithKline.Warner received a bachelors degree in commerce with Honors from the University of Saskatchewan.