The PMWC 2020 Clinical & Research Tools Showcase will provide a 15-minute time slot for selected companies in this space. The Clinical & Research Tools is a showcase for innovative technologies that are used for the analysis of genetic variation and function, helping to advance breakthroughs in genetic health, rare disease conditions on individuals of all ages, complex diseases and cancer care. Next-generation sequencing (NGS) and microarray technologies empower rapidly evolving genomic revolution.
Confirmed Presenting Companies:
Speaker Profile
Biography
Chhaya Shadra is an epidemiologist-informatician bringing her deep understanding of systems engineering and user workflow, to healthtech startups. Her recent work has focused on product development, architecture, data modeling, content harmonization, and algorithm development for integrated precision research data platforms sourced from EHR, claims, pharmacy, and medical device data. These platforms are currently being used by pharmaceutical companies in regulatory filings, health economic outcomes research(HEOR) and comparative effectiveness research(CER). Ms. Shadra’s architectural designs ensure interoperability of these custom datasets with standard CDMs like; i2b2, OMOP, Sentinel and PCORnet. The recent shift in focus of the FDA towards improved usage of real world datasets in the drug approval process makes Ms. Shadra’s work extremely relevant to the current healthcare landscape. Ms. Shadra has contributed to informatics and analytic engines at several health tech startups including but not limited to; Converge Health by Deloitte, Humedica by Optum and Flatiron Health.
Clinical & Research Tools Showcase:
Verana Health
Verana Health is assembling the largest clinical databases in medicine to empower physicians and accelerate research for patients. Our team partners with medical associations to assemble and activate these databases in Verana’s regulatory-grade data platform.
Key Considerations In the Design Of A Real World Research Data-Set
This presentation covers some best practices for a regulatory grade data-set from a data quality, interoperability and content standardization view-point in light of FDA’s guidance around Use of electronic health record data in clinical investigations.
Speaker Profile
Biography
Buzz Stewart has a long-standing interest in eliminating barriers to using data in real time to transform healthcare. During his more than 20 years of experience in healthcare delivery R&D, he has leveraged electronic health records and other sources of data to optimize healthcare delivery and scale precision medicine and clinal trails research. Buzz has designed and evaluated new models of care, developed streamlined process for biobanking, and used machine learning to develop predictive and phenotyping models. He is the co-founder and CEO of Medcurio, Inc, a start-up offering a seamless healthcare data access solution that eliminates the friction to getting and using data. He was previously Sutter Health’s Chief Research Officer (2012-2018), the founder of Geisinger’s Center for Health Research (2003-2012), VP of R&D, AdvancePCS (1998-2002), and faculty at the Johns Hopkins Bloomberg School of Public Health (1983-1995). Buzz received his Ph.D. from Johns Hopkins and MPH from UCLA.
Clinical & Research Tools Showcase:
Medcurio, Inc.
Medcurio, Inc. is a start-up that makes it easy to access, use, and learn from data in real time. Their platform enables non-technical users to develop and maintain APIs for real-time access to all healthcare data without writing any code.
Precision Medicine In Real Time, For Real!
Medcurio enables the delivery of precise data and guidance at the right time and place, for the right person, eliminating the headaches to getting and maintaining real-time data access. Real-word applications will be described.
Speaker Profile
Biography
Christian Apfel is internationally recognized for changing medical practice by bringing innovation to millions of patients by designing and executing on highest quality clinical research. One of his most notable innovations, the “Apfel Score,” is now used in clinical practice globally. Chris has turned his attention to precision medicine and is the founder and CEO of SageMedic Corporation. SageMedic redefines precision medicine with its SAGE Direct Platform™ to help millions of cancer patients to identify the most effective treatment by overcoming limitations inherent to genome-driven targeted therapy. He has published more than 100 peer-reviewed publications and is an Adjunct Professor at UCSF. He is an investor with Life Science Angels and the chair of the life science committee at the Keiretsu Forum. Chris is a California licensed physician and received his MD/PhD from Justus-Liebig-Universität Giessen, Germany, and his an MBA from Wharton, University of Pennsylvania, PA.
Clinical & Research Tools Showcase:
SageMedic Corp.
Our SAGE Direct Test™ enables oncologists to identify the most effective therapy for the millions of cancer patients where genomic testing fails to provide clinically useful guidance. We accomplish this by testing a drug panel on hundreds of live 3D microtumors created from the patient’s own biopsy in our own lab and with test results back in less than a week.
Overcoming Inherent Limitations Of Next-Generation Sequencing
Millions of cancer patients don’t get the most effective therapy because actionable genomic mutations are too rare. The only way to predict the most effective treatment is using functional profiling though previous attempts had their own limitations. In response, we developed the SAGE Direct Test™, which we expect to have more than 80% of accuracy in predicting the most effective treatment within a few days.
Speaker Profile
Biography
Dr. Henry’s research focuses on the molecular and cellular basis of cancer progression and metastasis in order to develop new methods for its diagnosis and treatment. His laboratory made the initial discovery that malignant cancer cells are resistant to brief pulses of fluid shear stress. Since then he has been exploring both the biological significance and practical applications of this finding. His work on both the mechanism and diagnostic potential of this finding as it relates to circulating tumor cells has been funded by the Department of Defense and the National Cancer Institute, including a Provocative Questions R21 grant. Dr. Henry has over 25 years of experience in cancer research in both academic and industry settings, serving as senior scientist with Millennium Pharmaceuticals prior to his current academic positions.
Clinical & Research Tools:
SynderBio
SynderBio is a life science instrumentation and diagnostic company that offers novel techniques to expedite and enhance cancer diagnosis and research efforts using our novel, targeted cell separation technology.
SynderBio's Rapid Cell Separation Technology For CancerDx
SynderBio has a unique approach to improving biopsy/sample clean-up that is significantly faster and greatly outperforms the competition in terms of yield, enrichment, and purity of the resulting sample. SynderBio has demonstrated consistent, effective sample preparation in a range of cancer cell types, outperforming FACS and MACS and disaggregating cell clusters while eliminating dead/dying cells to maximize retention of scarce clinical samples.
Speaker Profile
Biography
Dr. Zhou leverages experience in systems engineering, microfluidics and medical imaging for groundbreaking life sciences instrumentation. Before joining Triple Ring Technologies, Dr. Zhou was a pioneer in the development of a novel medical imaging modality, magnetic particle imaging. Dr. Zhou received a Ph.D. from the UC Berkeley-UCSF joint graduate group in bioengineering, and a B.S. in bioengineering from the University of Maryland, during which she coauthored 10 publications with over 160 citations.
Clinical & Research Tools Showcase:
Triple Ring Technologies
Triple Ring Technologies is an innovative research and development company that, in collaboration with clients & partners, develops technical solutions to critical, typically complex, challenges.
Decreasing QA/QC Costs Of Autologous Cellular Immunotherapy
QA/QC is a major cost of manufacturing autologous cell therapies due to the need for repeated QA/QC of a single patient dose. By automating common sterility and purity assays on an standalone system, we can make immunotherapy more accessible.
Speaker Profile
Biography
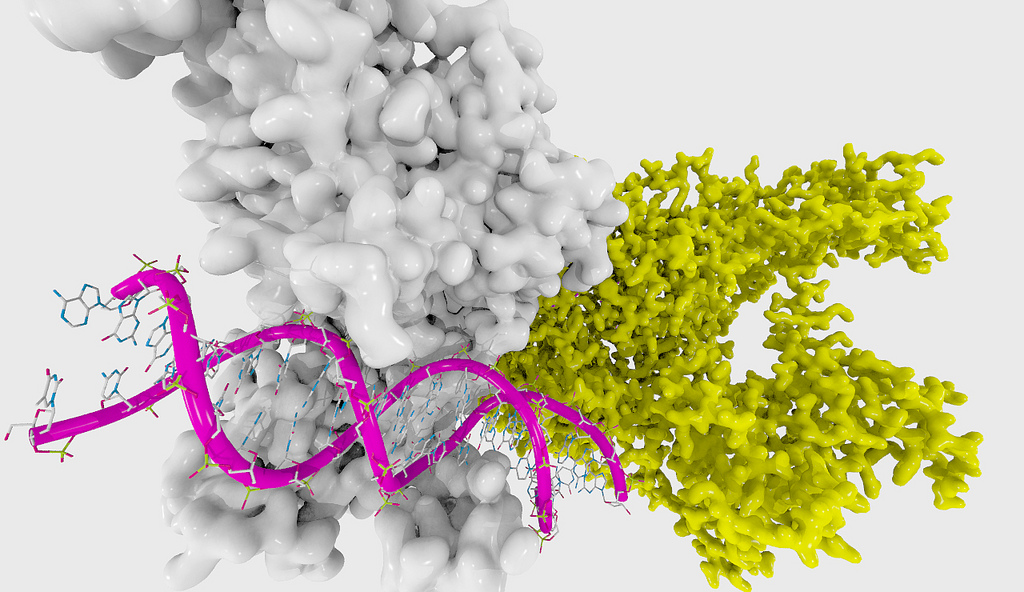
James is the CEO and co-founder of Precision NanoSystems, Inc. (PNI), a commercial-stage life science company at the convergence of gene therapy, nanotechnology, and advanced therapeutics. PNI provides comprehensive solutions for the development and manufacture of nanoparticle based Precision and Gene Therapies. This includes in the areas of RNA, DNA, CRISPR, small molecule, and protein therapeutics for the treatment of cancer, infectious disease, rare disease, cardiometabolic disease, and others. James earned a B.A.Sc. in engineering physics from UBC and a Ph.D. in genetics from the Institute for Systems Biology in Seattle, WA. James worked at the Seattle based Venture Capital firm, the Accelerator Corporation concurrent with his Ph.D. and has extensive experience in the science and commercialization of microfluidics, nanotechnology, and systems biology. James is a Director of Life Science British Columbia and an active participant of the bio-entrepreneurial community.
Clinical and Research Tools Showcase:
Precision NanoSystems
Precision NanoSystems (PNI) is a leading technology provider to drug developers for the discovery and manufacture of gene therapies and advanced therapeutics. PNI’s core technology includes its NanoAssemblr microfluidic-based continuous flow platform for nanomedicine development and manufacture, and its GenVoy nanoparticle delivery technology for nucleic acid delivery. PNI provides its technology to over 200 leading pharmaceutical companies, biotechnology companies, and academic institutions in over 20 countries worldwide.
Speaker Profile
Biography
Dr. Hung is an entrepreneur specializing in microfluidic platform technology development. He has 14 years of life science leadership experience in product development, R&D management, and bringing innovation from lab to marketplace. He was the co-founder and CTO of CellASIC, which was acquired by Merck KGaA, and served as the Head of Microfluidics in the Life Science Workflow division. He received his PhD in Electrical Engineering and Computer Science from UC Berkeley, and B.S. degree in Electrical Engineering from National Taiwan University.
Clinical & Research Tools Showcase:
COMBiNATi
The COMBiNATi |Q|&A platform is the only digital PCR platform with an identical workflow to traditional qPCR. Enhance your nucleic acid quantification without sacrificing precious sample, time, or reagents.
Introducing The |Q|&A Platform. Digital PCR Re-Imagined
COMBiNATi has developed the only dPCR system with a qPCR-equivalent workflow: one-step post-sample prep, no additional hands-on time, <90 minutes time-to-answer. With improved multiplexing and near-zero sample waste, COMBiNATi’s |Q|&A platform will change how you think about digital PCR.
Speaker Profile
Biography
William B. Dunbar (Bill) co-founded Ontera Inc. (formerly Two Pore Guys, Inc.) and currently serves as Chief Technology Officer to lead data analysis and IP creation and oversight for Ontera's technologies. Prior to Ontera, Bill was an engineering professor for 12 years at the University of California, Santa Cruz, publishing over 50 peer-reviewed papers and earning the National Science Foundation's prestigious CAREER award. Bill is co-inventor on 20 issued patents on diverse topics, from self-driving cars (owned by Toyota) to nanopore sequencing (licensed by Oxford Nanopore Technologies). Bill invented the dual nanopore device at UC Santa Cruz in 2011, and co-founded Ontera to license and commercialize that IP for applications that include genome mapping, structural variation analysis, epigenetics and long genome sequencing. Bill received a Ph.D. in control and dynamical systems theory from Caltech.
Clinical & Research Tools Showcase:
Ontera
Ontera's platforms use silicon nanopore chips and proprietary biochemistry to accurately detect and differentiate single molecules. We will help transform point of care testing and third generation sequencing.
Dual-Nanopore Mapping Of Epigenetic Features On DNA
Dual-nanopores are used to capture single DNA and repeatedly scan the molecule to generate electrical signal patterns that can be mined to map labeled sites of epigenetic relevance. Demonstrated labeling applications include base and histone modifications.
Speaker Profile
Biography
Dr Akrawi works in the field of biopharmaceutics and clinical pharmacokinetics, focused on bioavialability and bioequivalency studies for different molecules of drugs, drug-drug interaction, food-drug interaction, effects of drugs and some herbs on kidney and liver function, teaching and supervise under and post graduate pharmacy students. Dr Akrawi did researches in the stereoselective disposition of drugs and perform the portal continuous infusion of medication in unrestrained animal to study the stereoselective disposition of drugs and the effect of liver enzyme in the process. His PhD is from the college of pharmacy University of Kentucky.
Clinical & Research Tools Showcase:
King Faisal University
College of Clinical Pharmacy accredited by the American ACPE and Canadian CCAPP, Its program is to graduate Pharm D Pharmacist.
Development And Evaluation Of Mucoadhesive Buccal Film Of Almotriptan To Improve The Therapeutic Delivery
The aim of present investigation was to formulate oral mucoadhesive film of almotriptan to improve the drug delivery and desired therapeutic effects. Physicomechanical and pharmaceutical characteristics of drug loaded films (FA1-FA4) were examined. Selected FA4 film was evaluated in vivo by assessing the pharmacokinetic profile and compared with oral therapy in rabbits. In vivo data demonstrated rapid and efficient absorption (p<0.005) of almotriptan with greater AUC0-12 (>2 folds, p<0.0001) by FA4 film as compared to oral (control). Data established the potential of FA4 film to improve the therapeutic delivery of almotriptan and offers a promising option in migraine therapy.
Speaker Profile
Biography
Max is a Client Relations Manager at Aldevron focusing on CRISPR protein products and custom services since 2017. Prior to Aldevron, Max was a Business Development and Alliance Management Lead for BioTechnique, a GMP fill/finish CMO. Max holds a Bachelor’s Degree from the University of Wisconsin in Genetics and Life Sciences Communication.
Clinical & Research Tools Showcase:
Aldevron
Aldevron serves the biotechnology industry with custom production of nucleic acids, proteins, and antibodies. Thousands of clients use Aldevron-produced plasmids, RNA and gene editing enzymes for projects ranging from research grade to clinical trials to commercial applications.
Speaker Profile
Biography
Klaus Lindpaintner, MD, MPH, FACP previously held senior positions at Pfizer as Global Head, Human Genetics and Computational Biomedicine, at Hoffmann-La Roche, spearheading the company’s efforts in personalized health care; as CSO at Thermo-Fisher-Scientific; and on the faculty of Harvard Medical School. Klaus has co-authored some 250 scientific papers, and holds honorary and adjunct professor-ships at several academic institutions. He serves on numerous boards, working groups, and advisory panels for trade organizations, regulatory authorities, and non-governmental organizations.
Clinical & Research Tools Showcase:
InterVenn BioScience
InterVenn harnesses the elusive power of glycoproteomics for the discovery and development of high accuracy novel diagnostic, predictive, and prognostic markers as well as for discovery and validation of uniquely powerful new drug targets.
Harnessing The Power Of Glycoproteomics For Personalized Health Care
While genetics/genomics has facilitated impressive progress in the life sciences, its constraints based on the finite complexity and dynamic range of genomic data compared to the proteomeare becoming increasingly a limitation with regard to actionable clinical applicability. InterVenn has developed an AI-ML-empowered LC/MS platform that harnesses the vastly larger complexity and dynamic range of the proteome, in particular, the glycoproteome, and thus information content, rendering analytical performance of unprecedented accuracy.
Speaker Profile
Biography
Ana Oromendia has spent her career at the interplay of life sciences and technology, devoted to getting better treatments to the right patients at the right time. In her current role, she leads a team-building tools and technology to bring Omic data and analysis to Clinical Development, quickly and effectively enabling every clinical trial to collect Multi-Omic data and providing tools to ensure that all of the data is effectively analyzed. She has a background in experimental and analysis design of large 'omic datasets in large multidisciplinary scientific collaborations and the development of genetic tests and supporting software solutions. At Acorn AI a Medidata Company, Ana is part of a team creating analytical tools to improve the efficiency and rigor in the therapeutic development process and with the ultimate goal of getting more efficacious treatments to patients faster in the pursuit of precision medicine.
Clinical & Research Tools Showcase:
Medidata Solutions
Medidata helps generate the evidence and insights to help pharmaceutical, biotech, medical device and diagnostics companies, and academic researchers accelerate value, minimize risk, and optimize outcomes.
Executing Precision Medicine in Clinical Trials
Medidata’s Rave Omics Solution brings together data, technology, and expertise to ensure that you are getting the highest return on your Omic data investment. Machine learning and AI identify sample quality issues and accelerate the initial steps of biomarker discovery (real-world examples will be shared).
Speaker Profile
Biography
Nurul is the Head of Corporate and Research Development at Sengenics Corporation Pte Ltd, a functional proteomics company that leverages its patented KREX™ technology for production of full-length, correctly folded and functional proteins. Sengenics currently has partnerships with 9 out of the top 20 pharma to co-develop companion diagnostic tests for autoimmune and cancer immunotherapy drugs. Her previous experience in managing KREX operations has enabled her to take on her current role which focuses on accelerating KREX’s R&D pipeline towards FDA approval and commercialisation.
Clinical & Research Tools Showcase:
Sengenics
Sengenics is a functional proteomics company that leverages its patented KREX technology for stratification of patients undergoing treatment with autoimmune or cancer drugs into responders, non-responders and those exhibiting irAEs. KREX can also be used to identify autoantibody biomarkers for early diagnosis of cancer, autoimmune or neurodegenerative conditions.
Realising The Potential of Precision Medicine For Autoimmune And Cancer Drugs Using Sengenics' KREX Technology
Leveraging the Sengenics KREX™ technology, we have developed a fully quantitative protein array platform which enables the simultaneous screening of thousands of functional proteins, making it an ideal platform for the identification of autoantibody biomarkers in the development of in vitro diagnostic tests. This presentation will cover the key applications of our KREX™ technology and array derivatives towards enhancing precision medicine which include patient stratification, predicting treatment responses and biomarker discovery for early diagnosis of cancer and autoimmune diseases.