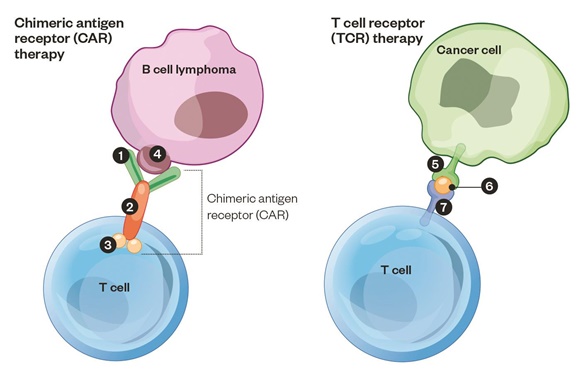
CAR-T cell therapy has evolved from an arcane approach under study in a handful of academic centers to a commercialized immunotherapy that is now being integrated into standard cancer care. The session will highlight some of the recent scientific advances that have begun to define major mechanisms of resistance to CAR-T cell therapy in B cell malignancies and identify the challenges that need to be addressed if these agents are to find broader applications in other hematological cancers and solid tumors.
Session Chair Profile
Biography
I focus on developing and applying NanoString platforms to address key research areas in immuno-oncology. As part of that mission, I work with academics, biopharmas, and clinicians to identify unmet needs in translational research and create novel products for transcriptional and proteomic profiling. I oversee the collaborations network for the company to help investigators utilize NanoString tools in their research with the goal of developing new biomarkers that can be deployed as clinical diagnostics. I am also active in the immuno-oncology research community to promote the science and application of cancer immunotherapy to improve patient outcomes. Prior to joining NanoString, I was a founder and director of research at Oncofactor Corp., a biotech focused on developing therapeutics which targeted novel immune checkpoints. I have a PhD in immunology from the University of Washington and a BS in biochemistry and English from Iowa State University.
Talk
Robust Transcriptomics to Inform Cellular Therapy During Manufacturing and Treatment
Speaker Profile
Biography
Dr. Melenhorst studies the biological basis of CAR T cell efficacy, including the immunobiology of CAR T cells, CAR engineering-related T cell reprogramming, and novel ways to augment the anti-tumor potency of tumor-redirected T cells. He was at the cusp of the first ever CAR T cell therapy approved by FDA: Kymriah and has contributed to seminal papers describing the clinical efficacy and CAR-related toxicities.
Talk
Biomarkers to Inform CAR-T Therapies
Proof of concept studies have demonstrated that chimeric antigen receptor (CAR)-reprogrammed T cells effectively target bulky dis-ease. We are discovering the natural basis of this form of immunogene therapy, and how synthetic biology further augments this pre-existing potency. My talk will cover both topics and discuss novel developments.
Speaker Profile
Biography
Sean Mackay is an experienced entrepreneur passionate about applying life sciences and information technology to improve healthcare delivery. He co-founded and leads IsoPlexis, a venture-backed startup focused on accelerating the fight against cancer using our predictive patient profiling platform. He has led the company through foundational licensing with Yale and Caltech, venture capital financing, and building out its fantastic team, while developing applications, products, and collaborations. Previously, he also helped incubate Kleiner Perkins-backed Lifesquare, which connected patients, payers, and providers through sharing essential healthcare information. At Lifesquare, he generated business development and strategic initiatives related to partnerships across the healthcare ecosystem. Through work at Lazard and with several early-stage ventures, he developed deep experience in structuring and financing life sciences and medical device and technology companies. Sean also helped multiple public and private biotechnology and medical technology companies to manage times of strategic change.
Talk
Single Cell Functional Proteomics To Accelerate Cell Therapy In Preclinical Development, Manufacturing, and Patient Difference Biomarkers
Speaker Profile
Biography
Dr. Posey is a geneticist proficient in the development and pre-clinical characterization of chimeric antigen receptors (CARs) and other engineered T cell strategies for cancer immunotherapy. He is developing novel CAR-T cell therapies that selectively targets a cell surface glycopeptides and glycoantigens found exclusively in cancers. The major objective of his research is to increase the efficacy of engineered T cells in solid tumors. Dr. Posey began his work in the CAR-T cell field in 2011. His initial work is currently being translated in a phase I clinical trial and he hopes to translate additional therapeutics in the next few years.
Talk
The Development Of Novel Cancer Therapies Targeting Abnormal Glycans For Humans
Speaker Profile
Biography
Dr. Ostertag directed Poseida’s spin out from Transposagen in February 2015 and has served as our chief executive officer and as a member of our board of directors since May 2015. From October 2003 to July 2015, Dr. Ostertag founded and served as the chief executive officer and president of Transposagen Biopharmaceuticals, Inc., a biotechnology company that commercializes early gene editing technology in the research reagent space. Dr. Ostertag previously co-founded and served as chief executive officer and president of Vindico NanoBioTechnology, Inc., a biotechnology company engaged in the discovery, development, and commercialization of human therapeutics that are based on a nanometer-scale particulate technology. Dr. Ostertag also co-founded and served as executive vice president of PhenoTech, Inc., a biotechnology company engaged in the discovery, development, and commercialization of reagents for diagnostic use in blood banks.
Talk
A Novel Approach To CAR-T Therapy
CART Therapeutics comprised of a high percentage of stem cell memory T cells exhibit unique properties that could potentially solve most of the limitations of early-generation CART products, including significant toxicities, historically poor efficacy against solid tumors, and high costs.
Speaker Profile
Biography
Dr. Miklos is a physician-scientist who has established a human translational immunology research group that fosters the development of both laboratory immunologists, and clinical translational researchers. His laboratory research focuses on 1) B cell reconstitution after allogeneic hematopoietic cell transplantation (alloHCT) 2) Lymphoid neoplasia minimal residual disease (MRD) quantification using Immune receptor high throughput sequencing technology 3) Prevention and treatment of chronic Graft versus host disease (cGVHD) and 4) CAR-T Cancer Cell Therapies. With his promotion to Associate Professor Spring 2016, he became Clinical Director of Stanford’s Cancer Immunotherapy program and Stanford’s Parker Institute for Cancer Immunotherapy (PICI). These aligned Immunotherapy clinical research groups focus on implementing human Chimeric Antigen Receptor Therapies (CAR-T), developing comprehensive clinical databases with linked biorepositories from industry sponsored and investigator initiated clinical trials. Dr. Crystal Mackall directs Stanford’s Center for Cancer Cell Therapy. Drs. Mackall and Miklos are two proven physician-scientists that synergize skills as pediatrician and adult oncologist that combine lab and clinical expertise daily. We have leveraged our comprehensive and established BMT program to provide Cancer Cell Therapy (CCT) clinician, research and laboratory expertise. Stanford CCT will advance cancer immunotherapy knowledge pursuing reiterative clinical trial research with biobanking and clinical databases to support innovative informative correlative studies including our bispecific CAR19-22 phase I trial and commercial CAR19 Therapies described herein.