Biography
Dr. Melenhorst studies the biological basis of CAR T cell efficacy, including the immunobiology of CAR T cells, CAR engineering-related T cell reprogramming, and novel ways to augment the anti-tumor potency of tumor-redirected T cells. He was at the cusp of the first ever CAR T cell therapy approved by FDA: Kymriah and has contributed to seminal papers describing the clinical efficacy and CAR-related toxicities.
Talk
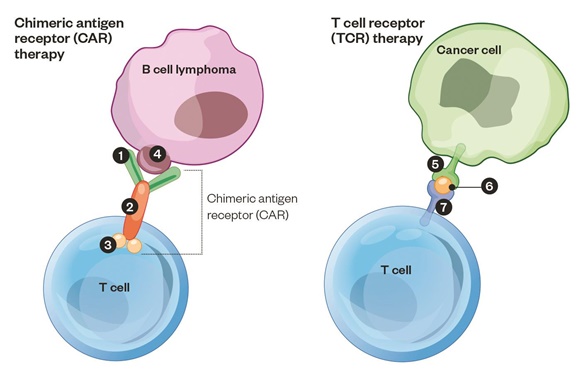
Biomarkers to Inform CAR-T Therapies
Proof of concept studies have demonstrated that chimeric antigen receptor (CAR)-reprogrammed T cells effectively target bulky dis-ease. We are discovering the natural basis of this form of immunogene therapy, and how synthetic biology further augments this pre-existing potency. My talk will cover both topics and discuss novel developments.
Session Abstract – PMWC 2020 Silicon Valley
CAR-T cell therapy has evolved from an arcane approach under study in a handful of academic centers to a commercialized immunotherapy that is now being integrated into standard cancer care. The session will highlight some of the recent scientific advances that have begun to define major mechanisms of resistance to CAR-T cell therapy in B cell malignancies and identify the challenges that need to be addressed if these agents are to find broader applications in other hematological cancers and solid tumors.