Biography
Wyllie is credited with pushing forward on the use of saliva as a superior sample for creating high-quality, low-cost testing. Having worked with saliva as a clinical specimen for bacterial detection for almost 10 years, Wyllie is an expert in the laboratory methods required for working with saliva and skillfully applied these for SARS-CoV-2 detection. Her innovation throughout the pandemic and her commitment to open science has led to the development of freely available PCR testing protocols that have enabled laboratories across the US to rapidly implement accessible SARS-CoV-2 testing programs. Coupled with public health guidance, Wyllie’s team has devoted their efforts to the safe re-opening of communities and to keeping kids in school. The use of saliva as a sensitive and reliable sample type can alleviate many of the bottlenecks encountered in the mass testing strategies required to control infectious disease outbreaks.
Clinical Dx Showcase:
Yale School of Public Health
SalivaDirect is more than a test protocol: it is a true community partner working to expand affordable, equitable COVID-19 testing solutions. We offer an innovative open-source PCR-based method for the clinical detection of SARS-CoV-2, while supporting local leaders navigating the pandemic response.
SalivaDirect: A Simplified and Flexible Platform for Accessible SARS-CoV-2 Testing
SalivaDirect™ was developed to increase access to testing while democratizing the testing process. This was achieved by challenging high testing costs and replacing rigid, expensive practices with a cost-effective, open-source protocol that allows for numerous substitutions along its streamlined process.
Session Abstract – PMWC 2022 Silicon Valley
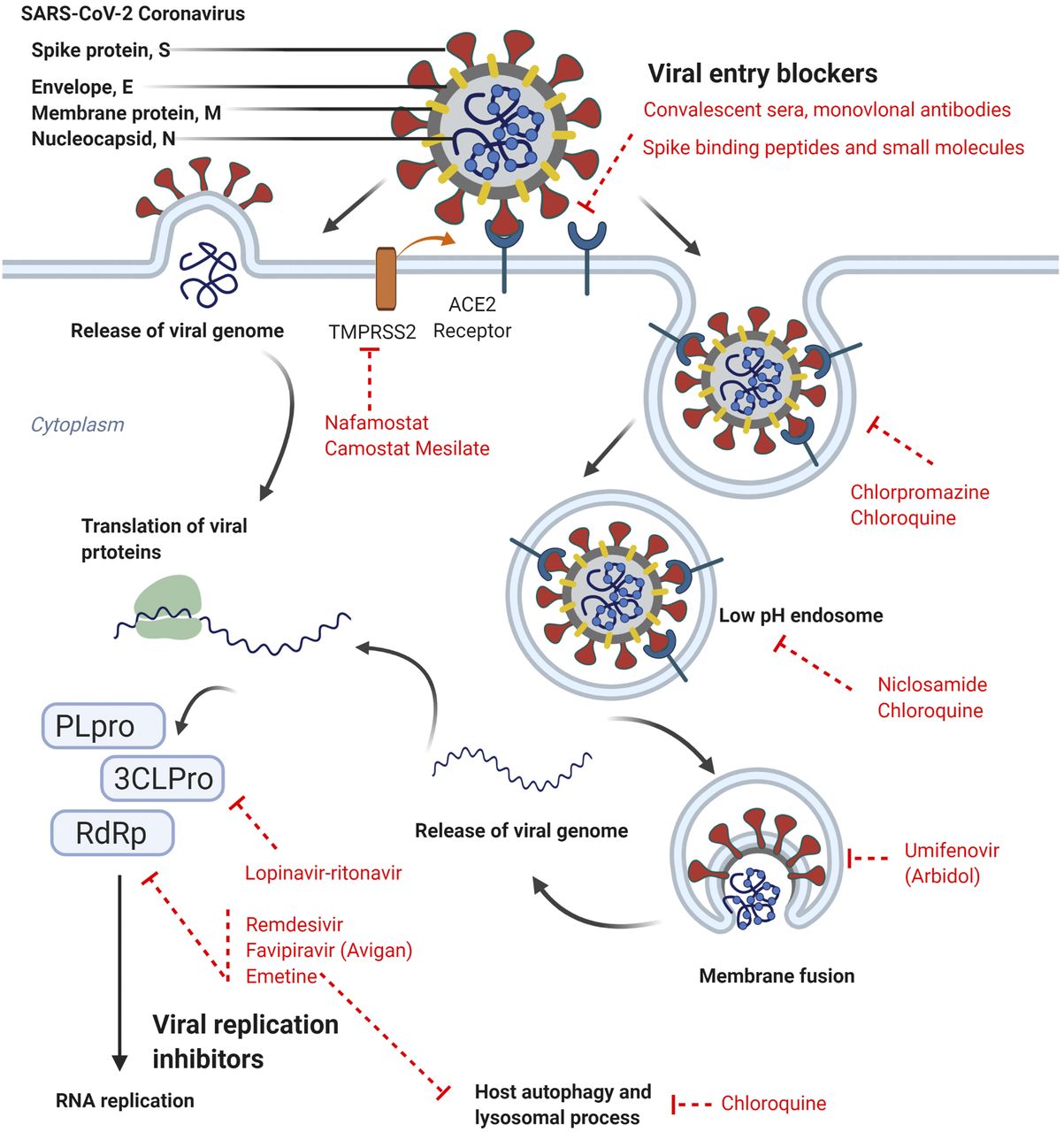
The COVID-19 pandemic is driving the unprecedented transformation of the global medical research ecosystem through the search for effective new therapeutics that can help ease symptoms and prevent death among COVID-19 patients.
- SARS-CoV-2 Outbreak Surveillance (PANEL)
Session Chair: Charles Chiu, UCSF
- Bronwyn MacInnis, The Broad Institute
- Duncan MacCannell, CDC
- Christopher E. Mason, Weill Cornell Medicine
- Joel Sevinsky, Theiagen - Experiences from the Front Lines of a National Viral Surveillance Organization
Session Chair: Erin Allday, The San Francisco Chronicle
- James Lu, Helix
- Sam Scarpino, Rockefeller Foundation - COVID-19 Testing: Who, When, What? The Challenges of Testing During the Pandemic (PANEL)
Session Chair: Alan Wu, UCSF
- Gary Pestano, Biodesix
- John Barnes, CDC
- Kathleen Jacobson, CA COVID Testing Task Force - Updates in COVID-19 Drug Development
Fireside Chat: Aida Habtezion, Pfizer & Lee Hood, Phenome Health
- Mark Dresser, Gilead Sciences
- John Fahy, UCSF
- Yi Shi, Mount Sinai
- Evolving SARS-CoV-2 Vaccines
- Daniela Weiskopf, La Jolla Institute for Immunology
- Karin Jooss, Gritstone - Accelerating COVID-19 Therapeutic Interventions
- Stacey Adam, FNIH
- Past, Present, and Future: How will We Manage the Next Pandemic? (PANEL)
Session Chair: Julianne McCall, California Initiative to Advance Precision Medicine
- Lawrence Corey, Fred Hutchinson Cancer Research Center
- Yvonne A Maldonado, Stanford
- Robert Wachter, UCSF
- Tomás Aragón, CDPH - PMWC Showcase
- Anne Wyllie, Yale