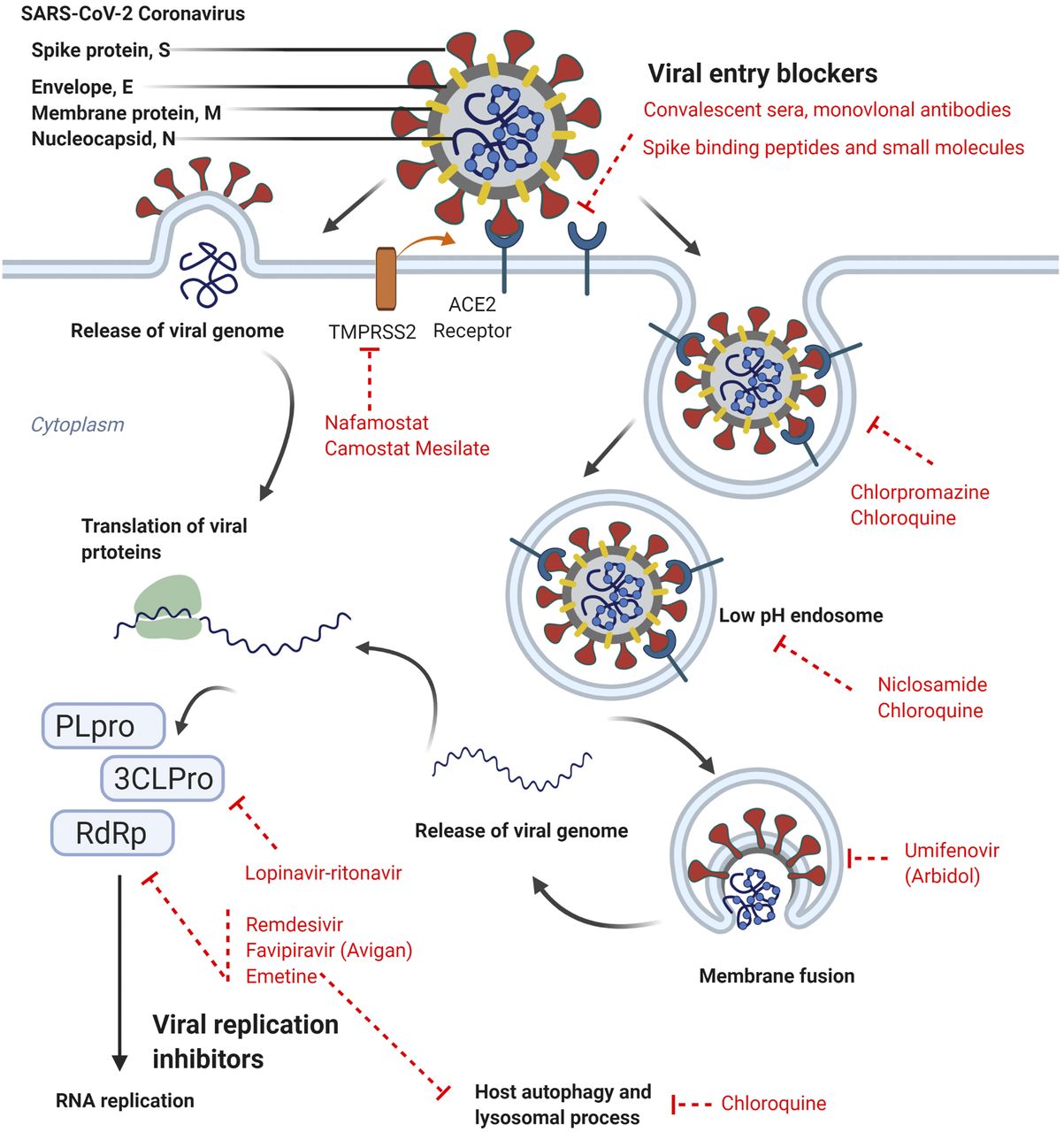
The COVID-19 pandemic is driving the unprecedented transformation of the global medical research ecosystem through the search for effective new therapeutics that can help ease symptoms and prevent death among COVID-19 patients.
- SARS-CoV-2 Outbreak Surveillance (PANEL)
Session Chair: Charles Chiu, UCSF
- Bronwyn MacInnis, The Broad Institute
- Duncan MacCannell, CDC
- Christopher E. Mason, Weill Cornell Medicine
- Joel Sevinsky, Theiagen - Experiences from the Front Lines of a National Viral Surveillance Organization
Session Chair: Erin Allday, The San Francisco Chronicle
- James Lu, Helix
- Sam Scarpino, Rockefeller Foundation - COVID-19 Testing: Who, When, What? The Challenges of Testing During the Pandemic (PANEL)
Session Chair: Alan Wu, UCSF
- Gary Pestano, Biodesix
- John Barnes, CDC
- Kathleen Jacobson, CA COVID Testing Task Force - Updates in COVID-19 Drug Development
Fireside Chat: Aida Habtezion, Pfizer & Lee Hood, Phenome Health
- Mark Dresser, Gilead Sciences
- John Fahy, UCSF
- Yi Shi, Mount Sinai
- Evolving SARS-CoV-2 Vaccines
- Daniela Weiskopf, La Jolla Institute for Immunology
- Karin Jooss, Gritstone - Accelerating COVID-19 Therapeutic Interventions
- Stacey Adam, FNIH
- Past, Present, and Future: How will We Manage the Next Pandemic? (PANEL)
Session Chair: Julianne McCall, California Initiative to Advance Precision Medicine
- Lawrence Corey, Fred Hutchinson Cancer Research Center
- Yvonne A Maldonado, Stanford
- Robert Wachter, UCSF
- Tomás Aragón, CDPH - PMWC Showcase
- Anne Wyllie, Yale
Session Chair Profile
Biography
Charles Chiu, M.D./Ph.D. is a Professor at UCSF, Director of the UCSF-Abbott Viral Diagnostics and Discovery Center (VDDC), and Associate Director of the UCSF Clinical Microbiology Laboratory. Chiu currently leads a translational research laboratory focused on clinical metagenomic sequencing assay development for infectious diseases and genomic investigation and surveillance of emerging pathogens, including the SARS-CoV-2 coronavirus. He also uses RNA-Seq transcriptome profiling to develop predictive models using machine learning for host response-based diagnosis of COVID-19 and other infections. Chiu’s work is supported by funding from the National Institutes of Health (NIH), Department of Defense, US Centers for Disease Control and Prevention (CDC), philanthropy, and the California Initiative to Advance Precision Medicine. He has authored more than 100 peer-reviewed publications (>20 on COVID-19), holds over 15 patents and patent applications, and serves on the scientific advisory board for Mammoth Biosciences, Danaher Dx, Biomesense, and Flightpath.
Session Chair Profile
Biography
Helix’s mission is to empower every person to improve their life through DNA. Helix is accelerating the integration of genomic data into clinical care and broadening the impact of large-scale population health programs by providing comprehensive expertise in DNA sequencing, bioinformatics, and individual engagement. Powered by our proprietary Exome+® assay, Helix offers health systems a scalable solution that enables the discovery of medically relevant, potentially life-saving, genetic Information. Prior to Helix, James was a faculty member at Duke University where he focused on translational genomics and machine learning methodologies for EMRs. James has explored research topics in population genetics, Mendelian genomics, and computational psychiatry.
Session Chair Profile
Biography
Dr. Wu's research involves pharmacogenomics, cardiac biomarkers and clinical toxicology. With the onset of the COVID-19 pandemic, he has switch his focus towards molecular testing for the virus, antigen and antibodies after an infection and after a vaccine. Throughout his career, he has been a leader in promoting the value of clinical laboratory testing to the medical world, but especially the lay public. During the early months of the pandemic, the absence lab testing availability was directly linked to the spread of the virus.
Session Chair Profile
Biography
Dr. McCall oversees cross-sector health policy working groups, research grantmaking, and public interagency efforts, which include serving on Governor Newsom's COVID-19 Testing Task Force and as co-author of the CA Surgeon General's Report. Previously, McCall worked at the California Senate Office of Research and as a Science and Technology Policy Fellow of the California Council on Science and Technology. Prior to state government, she spent sixteen years in neuroscience research labs, including as a Fulbright Fellow. In the community, Dr. McCall teaches Science Policy at UC Davis and UC Riverside, serves on the Editorial Board of the California Journal of Politics and Policy, occasionally directs the International Brain Bee, and is the co-founder of TEDxFulbright, the German Neuroscience Olympiad, and a chapter of the Sustained Dialogue Campus Network for racial justice. She earned a PhD from Heidelberg University, Master's degree from UC San Diego, and Bachelor's degree from Denison University.
Session Chair Profile
Biography
She most recently served as the chief scientific officer at Gritstone since 2016. Prior to Gritstone, from 2009 to 2016, Dr. Jooss was the head of cancer immuno-therapeutics at Pfizer, Inc., where she was also a member of the vaccine immuno-therapeutics leadership team. Prior to joining Pfizer, Dr. Jooss served as VP of research from 2005 to 2009 and as Sr. director of research at Cell Genesys Inc. from 2001 to 2005. She is on the editorial board of Molecular Therapy and the Journal of Gene Medicine and is a member of the Immunology and Educational Committee of the American Society of Gene & Cell Therapy. Dr. Jooss serves on the board of directors of Fate Therapeutics, Inc. Dr. Jooss received her diploma in theoretical medicine and a Ph.D. in molecular biology from the University of Marburg/Germany and performed postgraduate work in gene therapy and immunology at the University of Pennsylvania.
Talk
Self-Amplifying mRNA Multi-antigen Coronavirus Vaccine
The talk will cover the generation of chimeric antigen coronavirus vaccines to drive broad and potent T-cell responses against viral genes beyond Spike in addition to high neutralizing antibodies to Spike. The vaccine platform is a self-amplifying RNA (samRNA) which offers a potentially dose sparing vaccine opportunity to drive robust immune responses in humans.
Speaker Profile
Biography
Duncan MacCannell is the chief science officer for the CDC’s Office of Advanced Molecular Detection (OAMD), where he helps coordinate the implementation and support of pathogen genomics, bioinformatics, high-performance computing and other innovative laboratory technologies across the CDC’s four infectious disease centers. With a broad focus on public health laboratory science and strategic innovation, he helps to integrate standardized, sustainable capacity for advanced laboratory technologies and scientific computing into routine public health practice.
Speaker Profile
Biography
Bronwyn MacInnis's primary focus is on developing and implementing genomic epidemiology and surveillance approaches for malaria and viral pathogens, and building local capacity to integrate these into routine public health and clinical practice. Bronwyn co-leads the Broad’s large scale COVID-19 genomic surveillance effort, working closely with the Massachusetts and other State Departments of Public Health and the US Centers for Disease Control to monitor variants of concern and to track the evolution and spread of SARS-CoV-2 in New England and beyond. She also co-leads the Broad’s multidisciplinary Global Health Initiative and is a visiting scientist at the Harvard T.H. Chan School of Public Health. Her global health focuses on malaria genomic surveillance, outbreak prevention and response, and genomics capacity building.
Speaker Profile
Biography
Dr. Patrice Hugo is responsible for the medical affairs and scientific activities for the Central Laboratories, and Specialty Testing Centers of Excellence worldwide including the Expression Analysis Genomics, Vaccine, Biomarkers Translational Science and Innovation Laboratory and BioAnalytical/ADME laboratories. Prior to joining Q² Solutions, Dr. Hugo was Associate Vice President and Chief Clinical Trial Scientist at LabCorp/Covance Central Lab Division and Chief Scientific Officer at Clearstone Central Lab acquired by LabCorp. Previously, he worked at Caprion (now CellCarta) as Executive Vice President R&D, and Principal Investigator at the Montreal Clinical Research Institute with academic affiliation at McGill University and University of Montreal. Dr. Hugo obtained his Ph.D. in Experimental Medicine & Immunology at McGill University and held post-doctoral positions at the Walter and Eliza Hall Institute, Australia, and the Howard Hughes Medical Institute, USA. He is author or co-author of over 75 scientific manuscripts and has over 25 years of biomarker experience.
Speaker Profile
Biography
Dr. Pestano is an experienced leader in Product Development and clinical Laboratory Operations. Prior to joining Biodesix, Dr. Pestano held senior positions in Pharma Services, R&D and Project Leadership at Ventana Medical Systems, a member of the Roche Group. Dr. Pestano’s experiences are specialized in high complexity molecular diagnostics for oncology and virology and include molecular, serologic, and proteomic testing. Dr. Pestano is the co-inventor on multiple national and international patents for diagnostic tests. He has also fostered many collaborations in academia, industry, quality, and public health consortia as a part of new product development. Dr. Pestano received his Ph.D. in Molecular Cell Biology at The Graduate Center, City University of New York where his thesis focused on vaccine development for novel genetic variants of HIV-1. He conducted his post-doctoral training in Cancer Immunology and AIDS at the Dana Farber Cancer Institute, Harvard Medical School.
Talk
Real World Testing in Support of Back to Work Programs
Panelist providing real world perspective on multiple modalities of covid testing and studies in support of back to work programs.
Speaker Profile
Biography
Mark Dresser joined Gilead Sciences in 2020 as SVP, Biomarker Sciences and Clinical Pharmacology, where he oversees R&D efforts spanning biomarkers, diagnostics, clinical translational science, clinical pharmacology, pharmacometrics, and clinical bioanalysis. He has also served as Global Development Leader of Gilead’s 2nd Generation Remdesivir program. Mark was previously SVP and a founding member of the executive team at Denali Therapeutics, where he built numerous technical departments, including DMPK, Toxicology, and CMC. Under his leadership, Denali advanced five compounds into clinical development, and Mark also played a key role in the company’s successful IPO in 2017. He previously held scientific leadership positions at Genentech and Johnson & Johnson. In 2019, Mark received the UCSF Graduate Division Alumnus of the Year Award for his contributions to science and medicine. He completed his education and training at UCSF, Rensselaer Polytechnic Institute and the Swiss Federal Institute of Technology.
Speaker Profile
Biography
Dr. Larry Corey is an internationally renowned expert in virology, immunology and vaccine development, and the former president and director of the Fred Hutchinson Cancer Research Center. His research focuses on herpes viruses, HIV, the novel coronavirus and other viral infections, including those associated with cancer. He is principal investigator of the HIV Vaccine Trials Network, or HVTN, which conducts studies of HIV vaccines at over 80 clinical trials sites in 16 countries on five continents. Dr. Corey is also the principal investigator of the Fred Hutch-based operations center of the COVID-19 Prevention Network (CoVPN) and co-leads the Network’s COVID-19 vaccine testing pipeline. The CoVPN is carrying out the large Operation Warp Speed portfolio of COVID-19 vaccines and monoclonal antibodies intended to protect people from COVID-19.
Speaker Profile
Biography
John Fahy directs the UCSF Severe Asthma Faculty Practice and the UCSF Airway Clinical Research Center. His NIH supported research program aims to uncover disease mechanisms and to develop disease biomarkers and novel treatments for airway disease. His program is truly translational, encompassing clinical trials and investigations in patients and studies of disease mechanisms in cell culture and animal model systems. In clinical studies his lab aims to provide a molecular understanding of the clinical heterogeneity of airway disease and to explore new treatment options. In bench laboratory studies his lab focuses on how pathologic mucus forms in the airway in acute and chronic airway disease, on development of mucolytic drugs, and on mechanisms of persistent type 2 inflammation in asthma. In therapeutics he has specific interest and expertise in thiol drugs and he has applied this expertise to studies of thiol drugs as therapeutics for COVID19.
Talk
Thiol Drugs to Treat COVID19
Thiol drugs have anti-oxidant and anti-inflammatory properties that could limit SARS-CoV-2 lung injury or alter the redox status of the SARS-CoV-2 spike to disrupt ACE2 binding. We screened thiol drugs to show that they can act as virus entry inhibitors in vitro and modifiers of lung injury in hamsters.
Speaker Profile
Biography
My research has been focused on developing cutting-edge mass spec/proteomics technologies and protein bioengineering. Recently, we developed a robust proteomics pipeline to revolutionize nanobody (Nb)-drug discovery, and identified a large repertoire (> 8,000) of high-affinity, multi-epitope Nbs that target SARS-CoV-2 glycoprotein. Critically, we have demonstrated the outstanding preclinical efficacy of a stable, ultrapotent Nb for novel inhalation therapy of SARS-CoV-2 infection in a sensitive COVID-19 disease model. High-resolution cryoEM determination of Nb-bound structures reveals multiple potent neutralizing epitopes that target cryptic and conserved sites. Comparisons of neutralizing antibodies and Nbs provide insights into how Nbs target the spike to achieve high-affinity and broad activity. Structure-function analysis of Nbs indicates a variety of antiviral mechanisms. Our technologies will find broad utility in other devastating diseases including infectious diseases and cancer.
Talk
Inhalation COVID Therapy by Ultrapotent Nanobodies
Speaker Profile
Biography
Dr. Maldonado is a pediatric infectious diseases epidemiologist. She attended Stanford University School of Medicine, completed pediatric residency and pediatric infectious diseases fellowship at Johns Hopkins University, and was an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention before joining the faculty at Stanford University. She has led a number of NIH, CDC, Gates Foundation and WHO funded domestic and international pediatric infectious diseases studies, focused on pediatric HIV and pediatric vaccine preventable diseases. More recently, she has focused on health disparities and infectious diseases outcomes, including COVID-19 in pediatric and adult populations. She is chair of the American Academy of Pediatrics Committee on Infectious Diseases and a liaison to the CDC Advisory Committee on Immunization Practices. She has over 230 peer reviewed publications and is co-editor of the textbook “Infectious Diseases of the Fetus and Newborn Infant” and the American Academy of Pediatrics “Red Book”.
Speaker Profile
Biography
Stacey plays a leadership role at the FNIH, helping to lead many public-private partnerships, such as Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) the PPP that evaluated hundreds of available therapeutic agents with potential application for COVID-19, prioritized them, and designed and launched multiple master protocols to test them. Stacey oversees the Cancer, Metabolic Diseases and Clinical COVID Research portfolios at the Foundation for the National Institute of Health (FNIH). Beyond ACTIV other major partnerships under her guidance include the two steering committees of the Biomarkers Consortium and their projects, Partnership for Accelerating Cancer Therapies (PACT), and the Lung Master protocol (Lung-MAP) clinical trial. Prior to FNIH, she was a Manager at Deloitte Consulting within the Federal Life Sciences and Healthcare Strategy. She received her PhD at Duke and postdoctoral training at Stanford.
Talk
Agent Selection and Master Protocols for Evaluation of Candidate COVID-19 Therapeutics and Lessons Learned for Future Pandemics
Working in an unprecedented timeframe, the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership rapidly designed a unique therapeutic agent intake and assessment process for multiple types of candidate treatments (preclinical and clinical antivirals, immune modulators, SARS-CoV-2 neutralizing antibodies, and organ supportive) for COVID-19, reviewing over 800 and advancing over 30 agents to trials for testing. ACTIV also developed and launched ten master protocols between April 2020 and August 2021 to allow for the coordinated and efficient evaluation of these over 30 investigational therapeutic agents. The ACTIV master protocols were designed with a portfolio approach to serve multiple patient populations with COVID-19: outpatient mild to moderately ill, inpatient moderately ill, and inpatient critically ill and designed to test the spectrum of the disease pathophysiology. Each protocol, either adaptive or pragmatic, was designed to efficiently select those treatments that provide benefit to patients while rapidly eliminating those that were either not effective or safe. For both the agent prioritization and master protocol development ACTIV has captured lessons learned that may be useful in meeting the challenges of a future pandemic.
Speaker Profile
Biography
Robert M. Wachter, MD is Professor and Chair of the Department of Medicine at the University of California, San Francisco. He coined the term “hospitalist” in 1996 and is often considered the “father” of the hospitalist field, the fastest growing medical specialty in U.S. history. His 2015 book, The Digital Doctor: Hope, Hype and Harm at the Dawn of Medicine’s Computer Age, was a New York Times science bestseller. Thirteen times, Modern Healthcare magazine has ranked him as one of the 50 most influential physician-executives in the U.S.; he was #1 on the list in 2015. In 2020-21, his tweets on Covid-19 served as a trusted source of information on the clinical, public health, and policy issues surrounding the pandemic.
Speaker Profile
Biography
Dr. Sevinsky has leveraged over two decades of experience in systems biology and taken aim at transforming public health and infectious disease surveillance through innovative implementation of NGS and bioinformatics technologies. During a three year tenure at the Colorado Department of Public Health and Environment, he led several initiatives to build NGS testing and bioinformatics capacity at the state, regional and national levels. In 2019 Dr. Sevinsky’s ambitions to impact public health exceeded his role as a government scientist, resulting in the formation of Theiagen Genomics. Dr. Sevinsky and his team now work with over four dozen public health laboratories nationally, and more than a dozen internationally in Africa and Asia. Prior to his time in public health, Dr. Sevinsky held several positions in non-profit and industry, working in applied technologies for diverse applications such as biomarker identification and biofuel/biochemical production.
Speaker Profile
Biography
Striving to embody and promote the universal values of dignity, equity, compassion and humility, Dr. Aragón works to mobilize communities and institutions to transform policies and systems towards a culture of equity, antiracism, healing and health for all people and our planet. As State Public Health Officer, he exercises leadership and legal authority to protect health and prevent disease. As CDPH Director, he directs a nationally accredited state health department. Prior to coming to CDPH in January, 2021, he was the Health Officer of San Francisco for 10 years. He has served in leadership roles for more than 25 years, including principal investigator and director of a public health emergency preparedness and response research and training center at the UC Berkeley School of Public Health. On March 16, 2020, he and six other Bay Area health officers issued the first stay-at-home orders in the US.
Speaker Profile
Biography
Internationally recognized expert in the field of influenza viral genomics and diagnostics. Responsible for Manufacturing and Maintaining 6 FDA approved diagnostic assays including CDC’s Influenza A/B SARS-CoV-2 Multiplex Assay. Sequenced and published first RNA genome by sequencing the viral RNA directly. Team is one of the largest producers of influenza genomic data in the world.
Speaker Profile
Biography
Dr. Scarpino is an Affiliate Assistant Professor in the Department of Physics and holds appointments in the Network Science Institute, Institute for Experiential AI, Global Resilience Institute, and Roux Institute at Northeastern University. Scarpino has over 10 years of experience translating research into decision support and data science tools across diverse sectors from public health and clinical medicine to real estate and energy. From 2017-2020, he was Chief Strategy Officer and head of data science at the tech startup Dharma Platform. Scarpino has nearly 100 publications in academic journals and books. His commentaries on science and technology have appeared in publications such as: Nature, Science, PNAS, and Nature Physics. His research has been covered by the New York Times, Wired, NPR, VICE News, and other venues. He was made a Fellow of the ISI Foundation in 2017 and an External Faculty member of the Santa Fe Institute in 2020.
Speaker Profile
Biography
Dr Jacobson is a board-certified family physician and HIV/STD specialist. Prior to joining CDPH, Dr Jacobson was the Senior HIV TB Care and Treatment Advisor to the United States’ Center for Disease Control and Prevention (CDC) for the country of Uganda. Dr Jacobson was an Associate Professor of Clinical Family Medicine at the University of Southern California, Keck School of Medicine for over 20 years where she directed 3 medical school courses, was a principal investigator on a number of multisite studies on acute HIV, testing, rapid treatment, and linkage to care, and was the Medical Director of the Los Angeles Region AIDS Education and Training Center. She is also a Medical Officer with the US. Department of Health and Human Services which serves the Midwestern United States to Guam for National Disaster Management. Dr Jacobson has more than 27 years of clinical experience and has trained thousands providers in clinical care.
Speaker Profile
Biography
The Mason lab develops and deploys new biochemical and computational methods in functional genomics, and metagenomics. The group also works closely with NIST/FDA to build international standards. These methods are also being integrated for longitudinal multi-omic profiling of NASA astronauts and for genetic and epigenetic diagnostics on the International Space Station. Dr. Mason has won awards from the NIH, the CDC and the WorldQuant Foundation. He was named as one of the “Brilliant Ten” Scientists by Popular Science, and featured as a TEDMED speaker. His work represents 150+ peer-reviewed articles and has been featured on the covers of Nature, Science or Nature Biotechnology. Dr. Mason completed his dual post-doctoral training at Yale Medical School in genetics and a fellowship at Yale Law School. He obtained his Ph.D. in Genetics from Yale University, and his dual B.S. in Genetics and Biochemistry from University of Wisconsin-Madison.
Speaker Profile
Biography
As Chief Medical Officer of Pfizer, Dr. Habtezion leads Pfizer's Worldwide Medical & Safety organization responsible for ensuring that patients, physicians, and regulatory agencies are provided with information on the safe and appropriate use of Pfizer medications. She is a tenured and endowed Professor of Medicine, on a leave of absence from Stanford University who led translation research funded by multiple NIH, DOD, and foundation grants focused on understanding disease mechanisms in pancreatic and intestinal inflammatory diseases. She served as an Associate Dean for Academic Affairs in the School of Medicine, a faculty member in Stanford's Immunology Ph.D. program, Neuroscience Institute, Cancer Institute, Maternal & Child Health Research Institute, interdisciplinary biosciences institute Bio-X, and faculty fellow at Stanford's ChEM-H. She is the American Pancreas Association President, Fellow of the American Gastroenterological Association, an Allen Distinguished Investigator, elected member of the American Society for Clinical Investigation and Association of American Physicians.
Speaker Profile
Biography
Dr. Weiskopf, devoted her career to understanding the adaptive immune response to emerging infectious viruses relevant to human health and disease. Following her PhD, she obtained post-doctoral training at the La Jolla Institute for Immunology (LJI) where her efforts were dedicated to characterize human dengue virus-specific CD8+ and CD4+ T cell responses in samples from areas with endemic dengue infection and following experimental dengue vaccination. As a Research Assistant Professor in the Center for Infectious Diseases and Vaccine Research at LJI she have focused on the characterization of Zika and Chikungunya virus specific T cell responses and also became interested in the effects of pre-existing immunity against dengue virus to subsequent zika virus infection. Most recently she have characterized SARS-CoV-CoV-2 specific T cell responses after natural infection and vaccination. Understanding adaptive immune responses to SARS-CoV-2 is important for vaccine development efforts, interpreting disease pathogenesis, and calibration of future pandemic control measures.
Speaker Profile
Biography
Dr. Hood is currently on the faculty of the Institute for Systems Biology and CEO of Phenome Health, a non-profit organization driving the emergence of precision population health. He received an MD from Johns Hopkins Medical School and a PhD from Caltech. A professor at Caltech for 22 years, a co-founder and chairman of the first cross-disciplinary biology department at the University of Washington for 8 years; a co-founder and president of the Institute for Systems Biology for 18 years—the first organization promoting systems biology. He has co-founded 17 biotech companies; has 18 honorary degrees from leading institutions all over the world. He is in three US National Academies: Science, Medicine and Engineering. He developed the automated DNA sequencer that made the human genome sequence possible. He is pioneering data-driven and systems-driven 21st century medicine to transform healthcare.
Talk
Precision Population Health: A Million Person Project
Beyond the Human Genome (BHG), a second Human Genome Project, will analyze the genomes/phenomes for one million people over 10 years. BGH’s mission is to employ this data-driven approach to make a life of health accessible to all people. We are looking for partners from across the healthcare spectrum.
Speaker Profile
Biography
Wyllie is credited with pushing forward on the use of saliva as a superior sample for creating high-quality, low-cost testing. Having worked with saliva as a clinical specimen for bacterial detection for almost 10 years, Wyllie is an expert in the laboratory methods required for working with saliva and skillfully applied these for SARS-CoV-2 detection. Her innovation throughout the pandemic and her commitment to open science has led to the development of freely available PCR testing protocols that have enabled laboratories across the US to rapidly implement accessible SARS-CoV-2 testing programs. Coupled with public health guidance, Wyllie’s team has devoted their efforts to the safe re-opening of communities and to keeping kids in school. The use of saliva as a sensitive and reliable sample type can alleviate many of the bottlenecks encountered in the mass testing strategies required to control infectious disease outbreaks.
Clinical Dx Showcase:
Yale School of Public Health
SalivaDirect is more than a test protocol: it is a true community partner working to expand affordable, equitable COVID-19 testing solutions. We offer an innovative open-source PCR-based method for the clinical detection of SARS-CoV-2, while supporting local leaders navigating the pandemic response.
SalivaDirect: A Simplified and Flexible Platform for Accessible SARS-CoV-2 Testing
SalivaDirect™ was developed to increase access to testing while democratizing the testing process. This was achieved by challenging high testing costs and replacing rigid, expensive practices with a cost-effective, open-source protocol that allows for numerous substitutions along its streamlined process.