Biography
Dr. McCall oversees cross-sector health policy working groups, research grantmaking, and public interagency efforts, which include serving on Governor Newsom's COVID-19 Testing Task Force and as co-author of the CA Surgeon General's Report. Previously, McCall worked at the California Senate Office of Research and as a Science and Technology Policy Fellow of the California Council on Science and Technology. Prior to state government, she spent sixteen years in neuroscience research labs, including as a Fulbright Fellow. In the community, Dr. McCall teaches Science Policy at UC Davis and UC Riverside, serves on the Editorial Board of the California Journal of Politics and Policy, occasionally directs the International Brain Bee, and is the co-founder of TEDxFulbright, the German Neuroscience Olympiad, and a chapter of the Sustained Dialogue Campus Network for racial justice. She earned a PhD from Heidelberg University, Master's degree from UC San Diego, and Bachelor's degree from Denison University.
Session Abstract – PMWC 2022 Silicon Valley
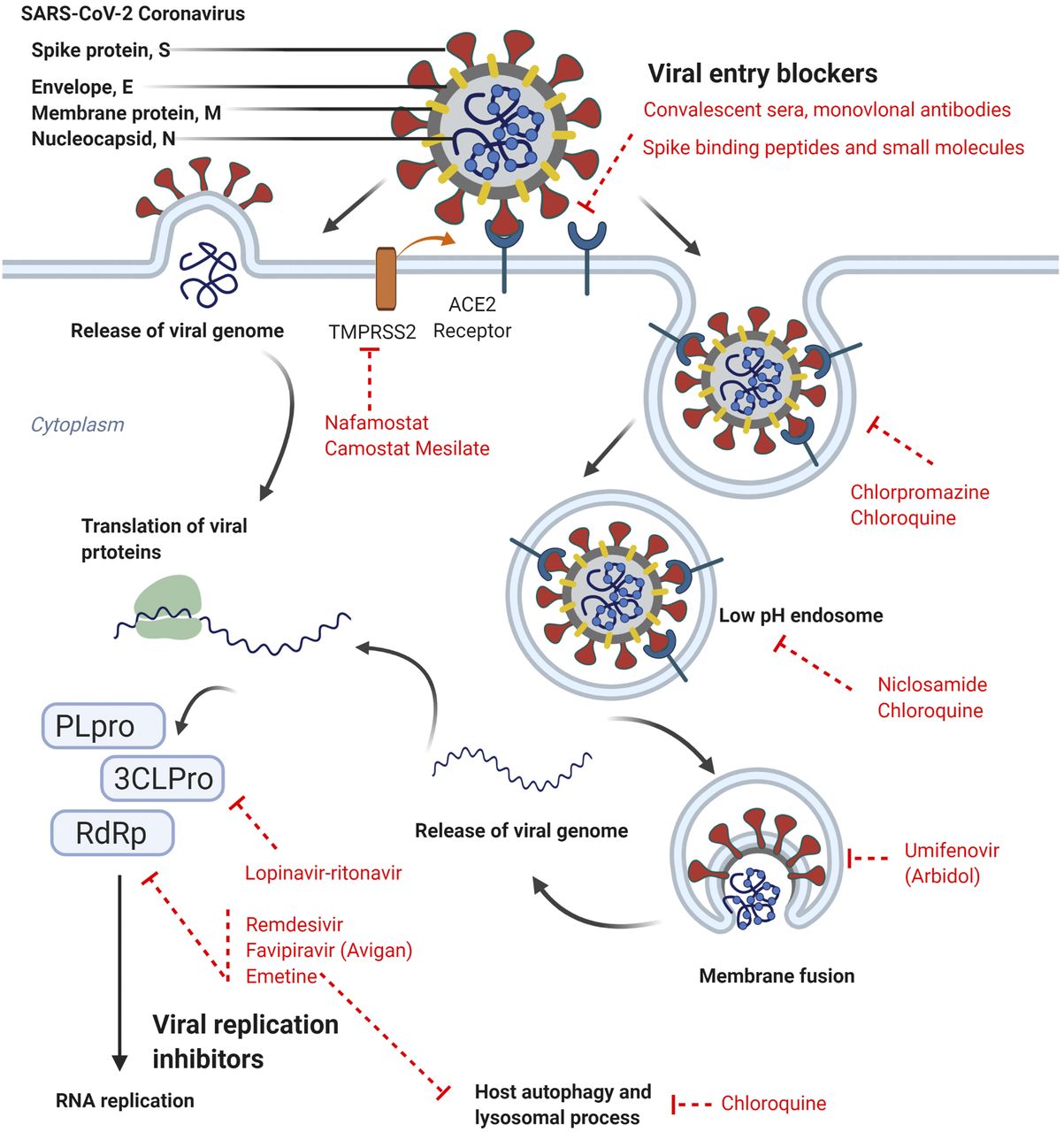
The COVID-19 pandemic is driving the unprecedented transformation of the global medical research ecosystem through the search for effective new therapeutics that can help ease symptoms and prevent death among COVID-19 patients.
- SARS-CoV-2 Outbreak Surveillance (PANEL)
Session Chair: Charles Chiu, UCSF
- Bronwyn MacInnis, The Broad Institute
- Duncan MacCannell, CDC
- Christopher E. Mason, Weill Cornell Medicine
- Joel Sevinsky, Theiagen - Experiences from the Front Lines of a National Viral Surveillance Organization
Session Chair: Erin Allday, The San Francisco Chronicle
- James Lu, Helix
- Sam Scarpino, Rockefeller Foundation - COVID-19 Testing: Who, When, What? The Challenges of Testing During the Pandemic (PANEL)
Session Chair: Alan Wu, UCSF
- Gary Pestano, Biodesix
- John Barnes, CDC
- Kathleen Jacobson, CA COVID Testing Task Force - Updates in COVID-19 Drug Development
Fireside Chat: Aida Habtezion, Pfizer & Lee Hood, Phenome Health
- Mark Dresser, Gilead Sciences
- John Fahy, UCSF
- Yi Shi, Mount Sinai
- Evolving SARS-CoV-2 Vaccines
- Daniela Weiskopf, La Jolla Institute for Immunology
- Karin Jooss, Gritstone - Accelerating COVID-19 Therapeutic Interventions
- Stacey Adam, FNIH
- Past, Present, and Future: How will We Manage the Next Pandemic? (PANEL)
Session Chair: Julianne McCall, California Initiative to Advance Precision Medicine
- Lawrence Corey, Fred Hutchinson Cancer Research Center
- Yvonne A Maldonado, Stanford
- Robert Wachter, UCSF
- Tomás Aragón, CDPH - PMWC Showcase
- Anne Wyllie, Yale
Session Abstract – PMWC 2022 Silicon Valley
Track Chair:
Keith Yamamoto, UCSF
Patient-centric data – Real-World Evidence (RWE) and Real-World Data (RWD) - is becoming instrumental in the drug development process and for health care decisions in general. This data is not only informative for the process from discovery to new indications, clinical trial design, and drug development, it also can be of value to monitor post-marketing drug safety and for decision support in clinical practice. As the data becomes a decision driver, science companies and medical organizations are increasingly focused on leveraging RWD and RWE to not only better understand the patient populations using their drugs and the respective outcomes, but also to accelerate clinical decision support. This session will focus on the various aspects of integrating RWE and RWD to support drug development and clinical decision support
Sessions:
- Realizing the Promise of Precision Medicine Using Real-world Evidence (PANEL)
Session Chair: Atul Butte, UCSF
- Nancy A. Dreyer, IQVIA
- Mark Laabs, RCRF
- Rhonda Cooper-DeHoff, University of Florida
- Chris Boone, Abbvie - Opportunities and Challenges in Using Real World Data (RWD) (PANEL)
Session Chair: Vivek Rudrapatna, UCSF
- Mark Hoffman, Childrens Mercy Hospital
- Ida Sim, UCSF
- Julian Hong, UCSF - How Are Patient Data Revolutionizing Precision Medicine? (PANEL)
Session Chair: Clara Lajonchere, UCLA
- Sharon Terry, Genetic Alliance
- Farid Vij, Invitae
- Latha Palaniappan, Stanford - Government Partnerships: California Initiative to Advance Precision Medicine
Session Chair: Julianne McCall, California Initiative to Advance Precision Medicine
- William Kim, UCSD
- Mike Snyder, Stanford - Leveraging RWE to Create Value (PANEL)
Session Chair: Latha Palaniappan, Stanford
- Brook Schroeder, Illumina
- Suzanne Belinson, Tempus
- Stacey Dacosta Byfield, Optum Genomics - Computational Social Choice Theory's Impact on the Collection and Understanding of RWD
- Ian Terry, LunaDNA - PMWC Showcase
- Chris Williams, MMRF
- Jason Crites, Assurance Health Data